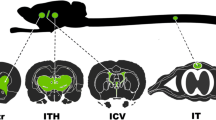
Abstract
Challenges in obtaining efficient transduction of brain and spinal cord following systemic AAV delivery have led to alternative administration routes being used in clinical trials that directly infuse the virus into the CNS. However, data comparing different direct AAV injections into the brain remain limited making it difficult to choose optimal routes. Here we tested both AAV9-egfp and AAV9-fLuc delivery via intrastriatal (IST), intracisterna magna (ICM) and lumbar intrathecal (LIT) routes in adult rats and assessed vector distribution and transduction in brain, spinal cord and peripheral tissues. We find that IST infusion leads to robust transgene expression in the striatum, thalamus and cortex with lower peripheral tissue transduction and anti-AAV9 capsid titers compared to ICM or LIT. ICM delivery provided strong GFP and luciferase expression across more brain regions than the other routes and similar expression in the spinal cord to LIT injections, which itself largely failed to transduce the rat brain. Our data highlight the strengths and weaknesses of each direct CNS delivery route which will help with future clinical targeting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Chowdhury EA, Meno-Tetang G, Chang HY, Wu S, Huang HW, Jamier T, et al. Current progress and limitations of AAV mediated delivery of protein therapeutic genes and the importance of developing quantitative pharmacokinetic/pharmacodynamic (PK/PD) models. Adv Drug Deliv Rev. 2021;170:214–37.
Merkel SF, Andrews AM, Lutton EM, Mu D, Hudry E, Hyman BT, et al. Trafficking of adeno-associated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J Neurochem. 2017;140:216–30.
Bailey RM, Rozenberg A, Gray SJ. Comparison of high-dose intracisterna magna and lumbar puncture intrathecal delivery of AAV9 in mice to treat neuropathies. Brain Res. 2020;1739:146832.
Murrey DA, Naughton BJ, Duncan FJ, Meadows AS, Ware TA, Campbell KJ, et al. Feasibility and safety of systemic rAAV9-hNAGLU delivery for treating mucopolysaccharidosis IIIB: toxicology, biodistribution, and immunological assessments in primates. Hum Gene Ther Clin Dev. 2014;25:72–84.
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–22.
Mathiesen SN, Lock JL, Schoderboeck L, Abraham WC, Hughes SM. CNS transduction benefits of AAV-PHP.eB over AAV9 are dependent on administration route and mouse strain. Mol Ther Methods Clin Dev. 2020;19:447–58.
Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20:1172–9.
Mattar CN, Wong AM, Hoefer K, Alonso-Ferrero ME, Buckley SM, Howe SJ, et al. Systemic gene delivery following intravenous administration of AAV9 to fetal and neonatal mice and late-gestation nonhuman primates. FASEB J. 2015;29:3876–88.
Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–69.
Jackson KL, Dayton RD, Klein RL. AAV9 supports wide-scale transduction of the CNS and TDP-43 disease modeling in adult rats. Mol Ther Methods Clin Dev. 2015;2:15036.
Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29:285–98.
Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M, et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther. 2011;19:1070–8.
Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34:204–9.
Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol Ther. 2018;26:664–8.
Taghian T, Marosfoi MG, Puri AS, Cataltepe OI, King RM, Diffie EB, et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol Ther. 2020;28:411–21.
Iannitti T, Scarrott JM, Likhite S, Coldicott IRP, Lewis KE, Heath PR, et al. Translating SOD1 gene silencing toward the clinic: a highly efficacious, off-target-free, and biomarker-supported strategy for fALS. Mol Ther Nucleic Acids. 2018;12:75–88.
Sinnett SE, Hector RD, Gadalla KKE, Heindel C, Chen D, Zaric V, et al. Improved MECP2 gene therapy extends the survival of MeCP2-null mice without apparent toxicity after intracisternal delivery. Mol Ther Methods Clin Dev. 2017;5:106–15.
Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. 2014;8:42.
Hordeaux J, Dubreil L, Deniaud J, Iacobelli F, Moreau S, Ledevin M, et al. Efficient central nervous system AAVrh10-mediated intrathecal gene transfer in adult and neonate rats. Gene Ther. 2015;22:316–24.
McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8:577–88.
Pietersz KL, Martier RM, Baatje MS, Liefhebber JM, Brouwers CC, Pouw SM, et al. Transduction patterns in the CNS following various routes of AAV-5-mediated gene delivery. Gene Ther. 2021;28:435–46.
Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206.
Bailey RM, Armao D, Nagabhushan Kalburgi S, Gray SJ. Development of Intrathecal AAV9 Gene Therapy for Giant Axonal Neuropathy. Mol Ther Methods Clin Dev. 2018;9:160–71.
Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19:852–9.
Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–9.
Hinderer C, Bell P, Vite CH, Louboutin JP, Grant R, Bote E, et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol Ther Methods Clin Dev. 2014;1:14051.
Pietersz KL, Martier RM, Baatje MS, Liefhebber JM, Brouwers CC, Pouw SM, et al. Transduction patterns in the CNS following various routes of AAV-5-mediated gene delivery. Gene Ther. 2021;28:35–446.
Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310.
Chang HY, Morrow K, Bonacquisti E, Zhang W, Shah DK. Antibody pharmacokinetics in rat brain determined using microdialysis. MAbs. 2018;10:843–53.
Chang HY, Wu S, Meno-Tetang G, Shah DK. A translational platform PBPK model for antibody disposition in the brain. J Pharmacokinet Pharmacodyn. 2019;46:319–38.
Hordeaux J, Buza EL, Dyer C, Goode T, Mitchell TW, Richman L, et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum Gene Ther. 2020;31:808–18.
High-dose AAV gene therapy deaths. Nat Biotechnol. 2020;38:910.
Matagne V, Borloz E, Ehinger Y, Saidi L, Villard L, Roux JC. Severe offtarget effects following intravenous delivery of AAV9-MECP2 in a female mouse model of Rett syndrome. Neurobiol Dis. 2021;149:105235.
Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–37.
Chatterjee D, Marmion DJ, McBride JL, Manfredsson FP, Butler D, Messer A, et al. Enhanced CNS transduction from AAV.PHP.eB infusion into the cisterna magna of older adult rats compared to AAV9. Gene Ther. 2021.
Palazzi X, Pardo ID, Sirivelu MP, Newman L, Kumpf SW, Qian J, et al. Biodistribution and tolerability of AAV-PHP.B-CBh-SMN1 in wistar han rats and cynomolgus macaques reveal different toxicologic profiles. Hum Gene Ther. 2022;33:175–87.
Chen X, Dong T, Hu Y, Shaffo FC, Belur NR, Mazzulli JR, et al. AAV9/MFSD8 gene therapy is effective in preclinical models of neuronal ceroid lipofuscinosis type 7 disease. J Clin Invest. 2022;132:e146286.
Castle MJ, Cheng Y, Asokan A, Tuszynski MH. Physical positioning markedly enhances brain transduction after intrathecal AAV9 infusion. Sci Adv. 2018;4:eaau9859.
Hinderer C, Bell P, Katz N, Vite CH, Louboutin JP, Bote E, et al. Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum Gene Ther. 2018;29:15–24.
Gurda BL, De Guilhem De Lataillade A, Bell P, Zhu Y, Yu H, Wang P, et al. Evaluation of AAV-mediated gene therapy for central nervous system disease in canine mucopolysaccharidosis VII. Mol Ther. 2016;24:206–16.
Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther. 2015;23:477–87.
Ramsingh AI, Gray SJ, Reilly A, Koday M, Bratt D, Koday MT, et al. Sustained AAV9-mediated expression of a non-self protein in the CNS of non-human primates after immunomodulation. PLoS One. 2018;13:e0198154.
Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, et al. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004;9:403–9.
Janelidze S, Nordstrom U, Kugler S, Brundin P. Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson’s disease. J Gene Med. 2014;16:300–8.
Pearson TS, Gupta N, San Sebastian W, Imamura-Ching J, Viehoever A, Grijalvo-Perez A, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat Commun. 2021;12:4251.
Funding
This work has been funded by Astrazeneca, UK.
Author information
Authors and Affiliations
Contributions
Experiment design: JC, TJ, GMT, MP, CD. Data analysis and interpretation: JC, EAC, DS, SW, GMT, MP. Manuscript preparation: EAC, JC, GMT. Supervision: GMT, MP, DKS, and IC.
Corresponding author
Ethics declarations
Competing interests
TJ, GMT, CD, DS, IC, and MP are employees of Astrazeneca, UK. JC is an employee of Asklepios Biopharmaceutical, Inc. EAC is an Astrazeneca sponsored postdoctoral fellow & DKS and SW are collaborators working at the University at Buffalo.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chandran, J., Chowdhury, E.A., Perkinton, M. et al. Assessment of AAV9 distribution and transduction in rats after administration through Intrastriatal, Intracisterna magna and Lumbar Intrathecal routes. Gene Ther 30, 132–141 (2023). https://doi.org/10.1038/s41434-022-00346-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-022-00346-1