Abstract
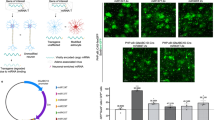
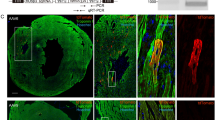
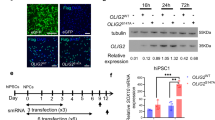
Biological rejuvenation by partial cell reprogramming is an emerging avenue of research. In this context, regulatable pluripotency gene expression systems are the most widely used at present. We have constructed a regulatable bidirectional adenovector expressing the humanized green fluorescent protein (GFP) and oct4, sox2, klf4, and c-myc genes (known as the Yamanaka genes or OSKM). The OSKM genes are arranged as a bicistronic tandem (hSTEMCCA tandem), which is under the control of a Tet-Off bidirectional promoter that also controls the expression of the gFP gene. Separately, a constitutive cassette expresses the regulatory protein tTA. Vector DNA was transfected in HEK293 Cre cells, which were additionally infected with the helper adenovector H14, unable to package its DNA due to the Cre recombinase produced by the HEK293 Cre cells. The newly generated vector was expanded by six iterated coinfections of the above cells which were lysed at the end of the process and the adenovector purified by ultracentrifugation in a CsCl gradient. The titer of the initial preparation was 1.2 × 1012 physical viral particles/ml. As expected, GFP fluorescence in vector-transduced rat fibroblast cultures declined with the dose of doxycycline (DOX) present in the medium. Immunocytochemical analysis of transduced cells confirmed the expression of the four Yamanaka genes. Additionally, 3 days after vector injection in the hypothalamus of rats, a significant level of fluorescence was observed in the region. Addition of 2 mg/ml DOX to the drinking water reduced the GFP expression. This adenovector constitutes a promising tool for implementing nonintegrative partial cell reprogramming.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell . 2006;126:663–76.
Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–74.
Oka K, Chan L. Helper-dependent adenoviral vectors. Curr Protoc Mol Biol. 2005;69(Suppl):16.24.1−23.
Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S. et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011;108:7838–43.
Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–22.
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–33.
López-León M, Outeiro TF, Goya RG. Cell reprogramming: therapeutic potential and the promise of rejuvenation for the aging brain. Age Res Rev. 2017;40:168–81.
Ma T, Xie M, Laurent T, Ding S. Progress in the reprogramming of somatic cells. Circ Res. 2013;112:562–74.
Kim J, Ambasudhan R, Ding S. Direct lineage reprogramming to neural cells. Curr Opin Neurobiol. 2012;22:778–84.
Maza I, Caspi I, Zviran A, Chomsky E, Rais Y, Viukov S, et al. Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotech. 2015;33:769–74.
Bar-Nur O, Verheul C, Sommer AG, Brumbaugh J, Schwarz BA, Lipchina I, et al. Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat Biotech. 2015;33:761–8.
Manukyan M, Singh PB. Epigenetic rejuvenation. Genes Cells. 2012;17:337–43.
Sánchez HL, Silva LB, Portiansky EL, Goya RG, Zuccolilli GO. Impact of very old age on hypothalamic dopaminergic neurons in the female rat: a morphometric study. J Comp Neurol. 2003;458:319–25.
Hereñú CB, Cristina C, Rimoldi OJ, Becú-Villalobos D, Cambiaggi V, Portiansky EL, et al. Restorative effect of Insulin-like Growth Factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Ther. 2007;14:237–45.
Morel GR, Sosa YE, Bellini MJ, Carri NG, Rodriguez SS, Bohn MC, et al. Glial cell line-derived neurotrophic factor gene therapy ameliorates chronic hyperprolactinemia in senile rats. Neuroscience. 2010;167:946–53.
Schwerdt JI, López-León M, Console GM, Brown OA, Morel GR, Spinedi EJ, et al. Rejuvenating effect of long-term IGF-I gene therapy in the hypothalamus of aged rats with dopaminergic dysfunction. Rejuvenation Res. 2018;21:102–8.
Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–9.
Ogorevc J, Orehek S, Dovč P. Cellular reprogramming in farm animals: an overview of iPSC generation in the mammalian farm animal species. J Anim Sci Biotechnol. 2016;7:10.
Salmon AB. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. AJP: Endocrinol Metab. 2005;289:E23–E29.
Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998.
Acknowledgements
The authors thank Dr. Gustavo Mostoslavsky, Boston University, for generous donation of the STEMCCA plasmid and Dr. Kazuhiro Oka, Baylor College of Medicine for donation of pLPBL-1. The authors are indebted to Mr. Mario R. Ramos for design of the figures, to Engineer Esteban Nigro for assistance with optical instruments and to Ms. Yolanda E. Sosa for editorial assistance. RGG, GRM, PCR, and CBH are Argentine National Research Council (CONICET) researchers. ML, MC-M, and PC are CONICET doctoral fellows.
Funding
This study was supported in part by grant PICT15-0817 from the National Agency for the Promotion of Science and Technology, from research grant MRCF 10-10-17 from the Medical Research Charitable Foundation and the Society for Experimental Gerontological Research, New Zealand to RGG and Grant M184 from National University of La Plata to CBH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lehmann, M., Canatelli-Mallat, M., Chiavellini, P. et al. Regulatable adenovector harboring the GFP and Yamanaka genes for implementing regenerative medicine in the brain. Gene Ther 26, 432–440 (2019). https://doi.org/10.1038/s41434-019-0063-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0063-x