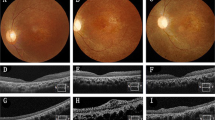
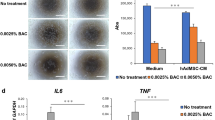
Abstract
Cell therapy has shown promising results for treating uveitis in preclinical studies. As the field continues to grow towards clinical translation, it is important to review and critically appraise existing studies. Herein, we analysed and critically appraised all preclinical studies using cell therapy or cell derived extracellular vesicles (EVs) for uveitis, and provided insight into mechanisms regulating ocular inflammation. We used PubMed, Medline, and Embase to search for preclinical studies examining stem cell therapy (e.g., mesenchymal stem cells [MSC]) and secreted EVs. All included studies were assessed for quality using the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) checklist. Sixteen preclinical studies from 2011 to 2022 were analysed and included in this review of which 75% (n = 12) focused only on cell therapy, 18.7% (n = 3) studies focused on EVs, and 6.3% (n = 1) study focused on both cells and EVs. MSCs were the most common type of cells used in preclinical studies (n = 15) and EVs were commonly isolated from MSCs (n = 3). Overall, both MSCs and EVs showed improvements in ocular inflammation (seen on fundoscopy/slit lamp and histology) and electroretinogram outcomes. Overall, MSC and MSC-derived EVs shown great potential as therapeutic agents for treating uveitis. Unfortunately, small sample size, risk of selection/performance bias, and lack of standardized cell harvesting or delivery protocols are some factors which limits clinical translation. Large scaled, randomized preclinical studies are required to understand the full potential of MSCs for treating uveitis.
摘要
细胞疗法在治疗葡萄膜炎的临床前研究中展示出良好的结果。随着这一领域不断向临床转化, 对现有研究进行回顾性和批判性地评估非常重要。在此, 我们分析并严格评估了使用细胞疗法或细胞产生的胞外囊泡 (EVs) 进行葡萄膜炎研究的临床前研究, 并深入探讨了调节眼部炎症的机制。我们使用PubMed、Medline和Embase检索了有关干细胞疗法 (如间充质干细胞 [MSC]) 和分泌的EVs的临床前研究。我们使用实验动物实验系统审查中心 (SYRCLE) 核对表对所有纳入的研究进行了质量评估。
本综述分析并纳入了2011年至2022年的16项临床前研究, 其中75% (n = 12) 研究只关注了细胞疗法, 18.7% (n = 3) 的研究关注了EVs, 而6.3% (n = 1) 的研究同时关注了细胞和EVs。间充质干细胞 (MSC) 是在临床前研究中使用最多的细胞类型 (n = 15), EVs通常从MSC中分离出来 (n = 3) 。总之, MSC和EVs对眼部炎症 (通过眼底镜/裂隙灯和组织学观察) 和视网膜电图结果均有改善。
总之, MSC和MSC衍生的EVs作为治疗葡萄膜炎的药物显示出了巨大的潜力。遗憾的是, 样本量小, 存在选择/选择偏倚的风险, 以及缺乏标准化的细胞采集或输送方案等因素限制了临床转化。需要进行大规模的、随机的临床前研究, 以了解MSC治疗葡萄膜炎的全部潜力。
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45:1–13.
Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23:705–17.
Babu K, Mahendradas P. Medical management of uveitis - current trends. Indian J Ophthalmol. 2013;61:277–83.
Zhao PT, Zhang LJ, Shao H, Bai LL, Yu B, Su C, et al. Therapeutic effects of mesenchymal stem cells administered at later phase of recurrent experimental autoimmune uveitis. Int J Ophthalmol. 2016;9:1381–9.
Li J, Qiu C, Zhang Z, Yuan W, Ge Z, Tan B, et al. Subretinal transplantation of human amniotic epithelial cells in the treatment of autoimmune uveitis in rats. Cell Transpl. 2018;27:1504–14.
Zhang L, Zheng H, Shao H, Nian H, Zhang Y, Bai L, et al. Long-term therapeutic effects of mesenchymal stem cells compared to dexamethasone on recurrent experimental autoimmune uveitis of rats. Investig Ophthalmol Vis Sci. 2014;55:5561–71.
Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366.
Kanda P, Davis DR. Cellular mechanisms underlying cardiac engraftment of stem cells. Expert Opin Biol Ther. 2017;17:1127–43.
Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884.
Li Y, Ren X, Zhang Z, Duan Y, Li H, Chen S, et al. Effect of small extracellular vesicles derived from IL-10-overexpressing mesenchymal stem cells on experimental autoimmune uveitis. Stem Cell Res Ther. 2022;13:100.
Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG, et al. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharm. 2020;11:590470.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Kung J, Chiappelli F, Cajulis OO, Avezova R, Kossan G, Chew L, et al. From systematic reviews to clinical recommendations for evidence-based health care: validation of Revised Assessment of Multiple Systematic Reviews (R-AMSTAR) for grading of clinical relevance. Open Dent J. 2010;4:84–91.
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–82.
Zhang X, Ren X, Li G, Jiao C, Zhang L, Zhao S, et al. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Investig Ophthalmol Vis Sci. 2011;52:3143–52.
Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Investig Ophthalmol Vis Sci. 2012;53:786–93.
Qin Y, Chan AM, Chang YL, Matynia A, Kouris NA, Kimbrel EA, et al. Human embryonic stem cell-derived mesenchymal stromal cells decrease the development of severe experimental autoimmune Uveitis in B10.RIII Mice. Ocul Immunol Inflamm. 2018;26:1228–36.
Oh JY, Kim TW, Jeong HJ, Lee HJ, Ryu JS, Wee WR, et al. Intraperitoneal infusion of mesenchymal stem/stromal cells prevents experimental autoimmune uveitis in mice. Mediat Inflamm. 2014;2014:624640.
Li G, Yuan L, Ren X, Nian H, Zhang L, Han ZC, et al. The effect of mesenchymal stem cells on dynamic changes of T cell subsets in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2013;173:28–37.
Lee HJ, Ko JH, Jeong HJ, Ko AY, Kim MK, Wee WR, et al. Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J Immunol. 2015;194:3634–45.
Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci USA. 2016;113:158–63.
Chen X, Shao H, Zhi Y, Xiao Q, Su C, Dong L, et al. CD73 pathway contributes to the immunosuppressive ability of mesenchymal stem cells in intraocular autoimmune responses. Stem Cells Dev. 2016;25:337–46.
Mu Y, Xu W, Liu J, Wang Y, Chen J, Zhou Q. Mesenchymal stem cells moderate experimental autoimmune uveitis by dynamic regulating Th17 and Breg cells response. J Tissue Eng Regen Med. 2022;16:26–35.
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, et al. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 2017;7:4323.
Kang M, Choi JK, Jittayasothorn Y, Egwuagu CE. Interleukin 35-Producing exosomes suppress neuroinflammation and autoimmune uveitis. Front Immunol. 2020;11:1051.
Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: Type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8:1214–25.
Caspi RR. Experimental autoimmune uveoretinitis in the rat and mouse. Curr Protoc Immunol. 2003;15:15.16.1–20.
Horai R, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011;31:733–44.
Foussat A, Gregoire S, Clerget-Chossat N, Terrada C, Asnagli H, Lemoine FM, et al. Regulatory T Cell therapy for uveitis: a new promising challenge. J Ocul Pharm Ther. 2017;33:278–84.
Muthu S, Bapat A, Jain R, Jeyaraman N, Jeyaraman M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021;8:7.
Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449.
Moller-Hansen M, Larsen AC, Toft PB, Lynggaard CD, Schwartz C, Bruunsgaard H, et al. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2021;19:43–52.
Calonge M, Perez I, Galindo S, Nieto-Miguel T, Lopez-Paniagua M, Fernandez I, et al. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl Res. 2019;206:18–40.
Ozmert E, Arslan U. Management of retinitis pigmentosa by Wharton’s jelly derived mesenchymal stem cells: preliminary clinical results. Stem Cell Res Ther. 2020;11:25.
Galderisi U, Peluso G, Di Bernardo G. Clinical trials based on mesenchymal stromal cells are exponentially increasing: where are we in recent years? Stem Cell Rev Rep. 2022;18:23–36.
Siqueira RC, Messias A, Voltarelli JC, Scott IU, Jorge R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina. 2011;31:1207–14.
Oner A, Gonen ZB, Sinim N, Cetin M, Ozkul Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Res Ther. 2016;7:178.
Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016;7:42.
Mastrolia I, Foppiani EM, Murgia A, Candini O, Samarelli AV, Grisendi G, et al. Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Transl Med. 2019;8:1135–48.
Kharbikar BN, Mohindra P, Desai TA. Biomaterials to enhance stem cell transplantation. Cell Stem Cell. 2022;29:692–721.
Author information
Authors and Affiliations
Contributions
PK was responsible for conception of study. PK, AG were responsible for designing the study protocol, screening studies, data acquisition, analysis, organizing tables/figures and drafting the manuscript. JD, DK were involved in data analysis, organizing tables/figures, and writing the manuscript. CG and PK was involved in helping revise the protocol, and editing manuscript. Final version of the manuscript was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanda, P., Gupta, A., Dhillon, J. et al. Mesenchymal stem cell based therapies for uveitis: a systematic review of preclinical studies. Eye (2024). https://doi.org/10.1038/s41433-024-03057-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-024-03057-6