Abstract
Glaucoma is the commonest cause of irreversible blindness worldwide, with over 70% of people affected remaining undiagnosed. Early detection is crucial for halting progressive visual impairment in glaucoma patients, as there is no cure available. This narrative review aims to: identify reasons for the significant under-diagnosis of glaucoma globally, particularly in Australia, elucidate the role of primary healthcare in glaucoma diagnosis using Australian healthcare as an example, and discuss how recent advances in artificial intelligence (AI) can be implemented to improve diagnostic outcomes. Glaucoma is a prevalent disease in ageing populations and can have improved visual outcomes through appropriate treatment, making it essential for general medical practice. In countries such as Australia, New Zealand, Canada, USA, and the UK, optometrists serve as the gatekeepers for primary eye care, and glaucoma detection often falls on their shoulders. However, there is significant variation in the capacity for glaucoma diagnosis among eye professionals. Automation with Artificial Intelligence (AI) analysis of optic nerve photos can help optometrists identify high-risk changes and mitigate the challenges of image interpretation rapidly and consistently. Despite its potential, there are significant barriers and challenges to address before AI can be deployed in primary healthcare settings, including external validation, high quality real-world implementation, protection of privacy and cybersecurity, and medico-legal implications. Overall, the incorporation of AI technology in primary healthcare has the potential to reduce the global prevalence of undiagnosed glaucoma cases by improving diagnostic accuracy and efficiency.
摘要
青光眼是全球最常见的不可逆失明性疾病, 超过70%的患者尚未确诊。目前尚无治愈方法。对于青光眼患者来说, 早期诊断对于阻止视力恶化至关重要, 本综述旨在: 找出全球范围内, 特别是澳大利亚普遍存在的青光眼患病率严重被低估的原因;以澳大利亚的医疗保健为例阐明初级医疗保健在青光眼诊断中的作用;讨论如何利用最新的人工智能 (AI) 技术来提高诊断的准确性。青光眼在人口老龄化的国家普遍存在, 通过适当的治疗可以改善视力结局, 因此对于医疗实践至关重要。在澳大利亚、新西兰、加拿大、美国和英国等国家, 验光师是初级眼科保健的守护者, 青光眼检测通常由他们负责。然而, 眼科专业人员在青光眼诊断能力上存在显著差异。利用AI对眼底彩色照片的视神经进行自动化识别技术可帮助验光师快速、高诊断效能地识别高风险变化, 并降低图像解读方面的挑战。尽管具有潜力, 但在将AI应用于初级保健场景之前, 还需要解决一些重要的屏障和挑战, 包括外部验证、高质量的实际应用、保护隐私和网络安全以及医疗法律问题。总之, 将AI纳入初级保健中将通过提高诊断准确性和效率来减少未确诊的青光眼病例的全球发病率。
Similar content being viewed by others
Introduction
Glaucoma poses a significant public health challenge. Despite its impact, a large percentage of glaucoma cases remain undetected, especially in primary eye care settings [1]. The primary objective of our review article is to explore the reasons behind the underdiagnosis of glaucoma, particularly in Australia, and discuss how recent advances in Artificial Intelligence (AI) applications can be utilised to improve diagnostic outcomes.
The review article will delve into the global significance of glaucoma and undetected cases, highlighting the prevalence of underdiagnosis in different regions, including Australia. We will examine the challenges faced by primary healthcare providers in accurately diagnosing glaucoma, such as the complexity of the diagnostic process and the lack of specialised training and equipment. Furthermore, we will discuss the potential of AI applications in addressing these challenges and improving glaucoma detection rates.
Our article will provide a comprehensive overview of the clinical detection of glaucoma, focusing on the use of digital imaging technologies, such as monoscopic fundus photos and optical coherence tomography (OCT), as well as the importance of visual field testing. We will also highlight the importance and challenges of glaucoma care at the primary eye care level, emphasising the roles of general practitioners (GPs) and optometrists in glaucoma diagnosis and management.
The section dedicated to AI applications will explore the potential of AI algorithms in glaucoma detection. We will present the performance of AI algorithms using fundus photography, OCT, and visual fields, showcasing their accuracy in diagnosing glaucoma. Additionally, we will discuss the deployment of AI products in clinical practice, addressing potential risks and the need for validation studies and protocols to ensure the reliability and safety of AI-assisted diagnosis.
Global significance of glaucoma and undetected glaucoma
The global significance of glaucoma and its underdiagnosis is a significant public health issue due to its status as the leading cause of irreversible blindness worldwide [2,3,4]. Glaucoma is the second leading cause of blindness among people aged 55 and over in Australia [5]. Despite its impact, over 70% of people with glaucoma remain undiagnosed globally [1]: 78%−94% for Africa [6], 72%−84% for Asia [7, 8], 57%−68% for Europe [9], 62%−78% for North America [10], 75%−88% for Latin America [11] and, 50%−60% for Australia and Oceania [7, 12,13,14]. The estimated numbers of glaucoma cases worldwide in 2020 are 52.7 million detected versus 43.8 million undetected. These numbers will increase in 2040 to 79.8 million and 67.1 million, respectively [1, 15].
The organisation of eye care services, skill levels of involved health care providers, and access to relevant health technologies contribute to glaucoma underdiagnosis. Organisational issues, particularly who provides eye care and who pays for it, varies globally. In Australia, the U.S., and many western European nations, routine eyecare is often fragmented between optometrists and primary healthcare physicians (GP), while ophthalmologists handle specialised patients and issues on referral from each. Inadequate diagnostic technology and provider training further exacerbate underdiagnosis. A new form of technology, Artificial Intelligence (AI), offers a potentially favourable transformation for glaucoma diagnosis. This promise, however, will depend on key financial, logistical, and organisational hurdles. The Australia example of diagnosing and managing glaucoma could be globally useful in addressing the significant public health issue of glaucoma underdiagnosis.
A non-systematic literature review was conducted based on articles published in peer-reviewed journals from the following databases: PubMed, oScopus, Medline-OVID, EMBASE, Cochrane Library, Web of Science and nongovernment organisation reports on 15 April 2023 using search terms to identify articles with no limitations on the publication year. The search combinations included “Glaucoma Care”, “Glaucoma Management”, “Glaucoma Diagnosis”, “Glaucoma Progression”, “Artificial Intelligence”, “Deep Learning”, “Machine Learning”, “Neural Networks”, “Bayesian Networks”, “Clinical Tools”, and “Primary Care”.
Overview of clinical detection of glaucoma
The hallmark of glaucoma is ganglion cell neurodegeneration in the optic nerve that is typically associated with functional loss. Consequently, diagnosis is largely based on optic nerve head (ONH) assessment and visual field (VF) testing, supplemented by several auxiliary tests and patient history (Table 1). Since the disorder is irreversible and has no available cure, early detection is vital for halting progressive visual impairment in glaucoma patients [16].
Detecting structural defects in glaucoma
Digital imaging technologies have become useful not only for documenting ONH and retinal nerve fibre layer (RNFL) changes (glaucomatous optic neuropathy or GON), but also for providing an objective, quantitative and convenient method to assist clinicians in glaucoma diagnosis.
Monoscopic fundus photos
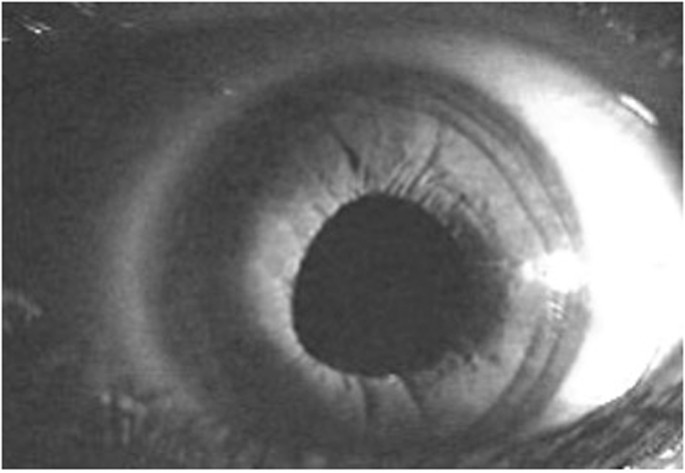
Accurate and consistent detection of the optic disc and RNFL due to progressive retinal ganglion cell death is the key to glaucoma diagnosis [17]. Traditionally, mydriasis is recommended to obtain a stereoscopic view for optic disc assessment. But a recent study shows that monoscopic optic disc photography provides non-inferior diagnostic accuracy for the clinical evaluation of all optic disc characteristics and glaucoma likelihood [18]. Monoscopic disc images offer a fast and affordable method to aid in GON detection.
Optical coherence tomography (OCT)
OCT is a non-contact, optical imaging technique that uses low coherence interferometry to measure backscatter from different layers of the optic nerve head and retina [19]. In a few seconds, it captures structural information such as RNFL thickness, ganglion cell layer thickness, disc size, and minimum rim width [20, 21] (the minimum rim width identifies the nerve fibre thickness passing out of the eye back to the brain). Pragmatically, longitudinal data are unlikely to be available to establish the initial glaucoma diagnosis. Therefore, structural information from a single observation (cross-sectional observation) could be considered as standard for diagnosis [20].
Detecting functional impairment in GON
Visual field testing
Visual sensitivity progressively declines in glaucoma. Standard automated perimetry (SAP) over the central 24°−30° of the visual field is the current recommended procedure for visual field testing in glaucoma [19, 22]. It measures retinal sensitivity to increments of light at many locations across the visual field. The 24−2 grid (central 24 indicates that the central 24 degrees of visual field and the next number indicates how the grid of points is aligned to the visual axis) is the preferred test strategy for this purpose, although the 30−2 is sometimes used to rule out other neurological conditions [23]. Repetition of visual field testing at baseline using the same threshold strategy is essential to establish a baseline so that the earliest presence of glaucomatous functional defect can be identified, and to accommodate for learning effects in performing the visual field test [24]. Visual field testing is recommended more than twice a year [25], with a minimum of three-times per year suggested to overcome test variability and identify progression in newly diagnosed glaucoma patients [26]. This frequency of testing will obviously challenge health care systems. Recent work indicates that a combination of structural and functional measures (VF and OCT) gives the greatest sensitivity for diagnosis and identifying progression in glaucoma [27].
Importance and challenges of glaucoma care at primary levels in Australia
Challenges faced by primary healthcare providers in glaucoma diagnosis
Glaucoma diagnosis is a complex process that requires targeted evaluation by eye care professionals. While primary healthcare providers play an important role in glaucoma screening, they face numerous challenges in accurate diagnosis. The diagnostic process includes history taking, assessment of the ONH and RNFL, measurement of intraocular pressure (IOP), evaluation of the anterior angle and anterior chamber by slit lamp to exclude angle closure or secondary causes of glaucoma, and VF evaluation. Although these assessments are essential for definitive glaucoma diagnosis, not all are required for screening to identify presumptive glaucoma (Box 1).
A study conducted in Victoria, Australia, found that out of 4744 individuals, 72 cases of referable glaucoma were identified, of whom 35 (49%) were undiagnosed [1]. The primary cause of misclassification was the lack of VF screening, as 97% of their 35 missed cases failed a VF screening test during a subsequent date. The study also found that 66% of the missed cases had an enlarged cup-to-disc ratio, which was not identified during their previous eyecare visits [1]. Perhaps this finding is not unexpected as cup-disc evaluation using ophthalmoscopy is challenging, and GPs are not versed in performing this procedure, so they may not identify abnormality from normal variations. However, in the case of this study, most participants who had enlarged cup-disc ratios had seen an optometrist or an ophthalmologist in the past year, and their physical state did not provide adequate evidence for further evaluation. This implies that cup-disc evaluation is a complex and challenging undertaking even for ophthalmic practitioners and would likely lead to high false positive or false negative outcomes if undertaken by GPs.
The implementation of AI has the potential to alleviate some of the challenges faced by primary healthcare providers in glaucoma diagnosis Box 2. AI technology can aid in the interpretation of images of the ONH, RNFL, and VF, providing a more accurate and consistent assessment. However, before AI can be widely adopted in primary healthcare settings, several issues must be resolved first, as we will discuss later in this article.
A case of primary eye care in glaucoma diagnosis in Australia
Australia’s healthcare system is founded on the principles of universal health care and a robust public insurance programme, ensuring that all citizens and permanent residents and New Zealand citizens have access to free and high-quality medical services [28]. This fundamental aspect has played a pivotal role in the successful implementation of national cancer screening programmes for bowel, cervical and breast cancer [29]. The achievements of these screening programmes underscore the capability of Australia’s healthcare system in effectively executing universal screening initiatives. Considering the accomplishments in cancer screening, there is a strong foundation for the feasibility of developing and executing a universal glaucoma screening programme. The existing infrastructure, along with the commitment to preventive healthcare, positions Australia favourably to address glaucoma detection and management comprehensively. By leveraging its healthcare system’s strengths and incorporating the latest advancements in medical technology, Australia has the potential to enhance the early diagnosis and treatment of glaucoma, ultimately safeguarding the vision and overall health of its population.
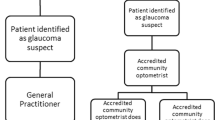
In Australia there are theoretically two models of primary care for glaucoma detection—by GPs and by optometrists. In practice, however, GPs are not trained to diagnose glaucoma and do not have access to testing equipment, therefore the burden of glaucoma diagnosis falls to optometrists. However, optometrists may face challenges in performing all the required procedures for glaucoma diagnosis (Table 1), especially when under time pressures, as experienced by all primary health care practitioners.
Starting in 2009, topical glaucoma medications prescribed by optometrists became available at subsidised prices (Pharmaceutical Benefits Scheme, PBS) [30]. In 2020, there were 6043 registered optometrists in Australia, with 65% having prescribing endorsement [31]. Despite being given increasing independence in prescribing [32], optometrists have not widely embraced this role. In 2015, latanoprost was the drug most prescribed by optometrists under PBS. However, while about 40% of these optometry prescriptions are for glaucoma management, they only accounted for 1.3% of all glaucoma prescriptions [32]. There were around 1000 full-time equivalent (FTE) ophthalmologists and 4800 FTE optometrists employed in Australia in 2019, or 4 FTE ophthalmologists and 19 FTE optometrists per 100,000 population [25]. Thus, optometrists were not highly active in managing glaucoma with most cases referred to ophthalmology.
Current glaucoma care guidelines for optometry
A patient who presents to an optometrist with glaucoma risk factors during a routine eye examination is recommended to undergo further clinical investigations to determine if glaucoma is present. For this purpose, the Optometry Board of Australia guidelines [33] recommend that practising optometrists have certain equipment to form a proper evaluation and differential diagnosis (Table 1). If this equipment is not available, or if testing cannot be undertaken to satisfy the requirements of Table 1, then a referral should be made to another optometrist or ophthalmologist for specialised testing and interpretation of the test results.
The glaucoma assessment needs to be made by all optometrists, whether endorsed for the use of scheduled medicines (therapeutically endorsed) or not. If an optometrist is therapeutically endorsed, they are authorised to diagnose and initiate glaucoma treatment independently. Once the diagnosis is established and the treatment started, patients are to be seen by an ophthalmologist within 4 months [33]. This referral reflects that surgical intervention is sometimes a viable first-choice treatment option and should be considered in all cases [34, 35].
Although this surgical related review produces a best-case option, delays in seeing an ophthalmologist can be substantial due to high caseloads [31]. A patient must be referred by a primary care provider (optometrist or GP) to gain access to specialist eye care in a public hospital in Australia and to be eligible for a rebate under the national health insurance act (Medicare Benefits Schedule) [36]. A 2020 study found that 72% of glaucoma referrals to the public hospital system came from optometrists [37]. However, the study also found that the median wait-time for patients to be seen by an ophthalmologist at the hospital can be as long as 400 days [37]. This delay in treatment can lead to irreversible sight loss, which can be avoided if optometrists/GPs prescribe IOP reducing drugs on diagnosis, given that most optometrists are therapeutically qualified. It will also act to minimise the possibility of vein occlusion associated with lengthy exposure to elevated IOP. Patients can suffer harm and sight loss due to 22 week delay in achieving hospital attendance [38], which supports an argument for therapeutic intervention on diagnosis to reduce the potential for sight loss prior to hospital attendance.
Why are so many people with glaucoma not diagnosed?
The cause of the high prevalence of undetected glaucoma is multifactorial [39,40,41]. Firstly, as above, glaucoma diagnosis requires the consideration of complex diagnostic tests, many of which are not available to GPs and some optometry settings. Additionally, glaucoma (except for acute angle closure glaucoma) is hard to diagnose as patients have few or no symptoms of the disease [4]. Visual symptoms are rare in early and middle stages of glaucoma, thus glaucoma is coined “the silent thief of vision”[1].
Secondly, while universal screening for glaucoma is not cost-effective in Western countries [42], detection of glaucoma relies on primary care providers, namely optometrists and GPs. However, GPs rarely test patients for glaucoma due to a lack of training and specialised equipment [43]. Fortunately in Australia, we have universal health coverage for routine comprehensive eye examinations in optometry settings, and all optometry students are trained in glaucoma care. However, variation exists in ONH assessment, which could have contributed to the high rate of undetected glaucoma reported in Australia [44].
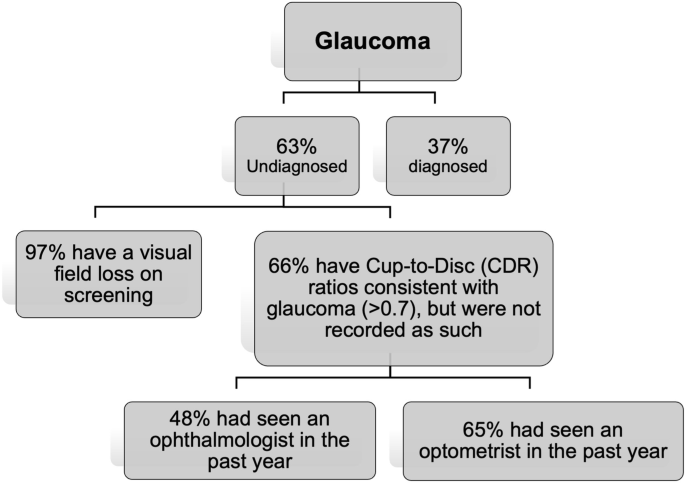
A population study [1] in Victoria found that undiagnosed glaucoma was as high as 63% (Fig. 3). Of the undiagnosed cases, 66% had seen an optometrist in the past year, 97% did not have a visual field test performed, and 66% had Cup-to-Disc (CDR) ratios consistent with glaucoma (>0.7) but were not recorded as such. Of note, out of those undiagnosed cases with CDR > 0.7, 65% had seen an optometrist and 48% had seen an ophthalmologist in the past year [1]. This indicates that glaucomatous disc changes are challenging to detect, which may explain the significant missed diagnosis. The prevalence of undiagnosed disease is even higher in minority populations [45, 46].
Undiagnosed glaucoma in Victoria, Australia (calculated on data from Wong et al. [1]).
Standardising the detection of GON based on structural and functional changes can help bridge the gap in glaucoma detection. AI can potentially play a useful role in this regard by providing consistent imaging interpretation and identifying high risk cases for closer consideration by the optometrist.
Potential of AI (artificial intelligence) in glaucoma diagnosis in primary eye care settings
Context and definition
When Deep Blue (a chess-playing computer developed by IBM [47]) defeated Garry Kasparov (the youngest world chess champion in history) in 1997, the defenders of human supremacy moved humanity’s battleground to Go (an abstract strategy board game for two players in which the aim is to surround more territory than the opponent. The number of legal board positions in Go has been calculated to be approximately 2.1 × 10170 [48], which is far greater than the number of atoms in the observable universe, estimated to be of the order of 1080) [49]. Piet Hut, an astrophysicist and Go enthusiast, predicted that it would take “a hundred years before a computer beats humans at Go—maybe even longer.” But in just under 20 years since Deep Blue vs. Kasparov, a computer programme developed by neuroscientist and chess prodigy Demis Hassabis and his DeepMind team surpassed all human players at Go [50].
As in other domains, AI is rapidly changing the healthcare landscape. Recent advancements in AI have introduced promising opportunities for efficient and cost-effective glaucoma detection programmes. Many studies have shown that AI algorithms now equal or exceed expert diagnostic accuracy for many conditions, particularly when the diagnosis is based on image interpretation, such as in dermatology and radiology [51,52,53,54,55,56,57]. Eye care is the frontrunner of the AI revolution in health care because diagnosing eye conditions heavily depends on imaging. In 2018, IDx-DR (Digital Diagnostics), designed to detect diabetic retinopathy and diabetic macular oedema, became the first FDA-approved autonomous AI device in any field of medicine [58]. The integration of AI into the glaucoma diagnostic process has the potential to significantly reduce costs and resource burdens and provides a potential to yield more accurate diagnosis.
Overview for performance of AI algorithm in glaucoma detection
AI using fundus photography
AI can help address the issue of variation in the assessment of ONH and RNFL changes. Segmentation and structured learning from various studies achieved an accuracy between 94% and 98% [59, 60] in reaching a correct diagnosis from fundus photos in glaucoma. Various deep learning algorithms based on fundus features such as the cup-to-disc ratio achieved area under receiver operating curve (AROC) between 0.53 and 0.996 in differentiating healthy from glaucomatous eyes [61,62,63,64], with a sensitivity ranging from 96% to 100% [61, 65], and specificity of 98% [64, 65]. Recently, Machine Learning (ML) algorithms developed from the interrogation of 50,000 fundus photos achieved an area under curve (AUC) of 0.986 with 95.6% sensitivity and 92% specificity for identifying referable GON [66]. AI-based tools can provide standardised and objective assessments, leading to more accurate and consistent diagnoses.
AI using OCT
The RNFL thickness is one common parameter utilised for glaucoma diagnosis [67] and became a key focus in ML using OCT images. Since 2005, studies have reported the performance of ML algorithms analysing OCT imaging data from peripapillary RNFL thickness maps and the macular ganglion cell complex for detecting GON, with AROC values ranging from 0.69 to 0.99 [68,69,70,71,72,73,74,75]. A recent study showed that Deep Learning (DL) network achieved an AROC of 0.94 for detecting GON using unsegmented OCT volumes of the optic nerve head [76]. Given the cost of OCT this application of AI is likely best retained for specialist or optometry practice where ocular OCT can be applied for many other purposes.
AI using visual fields
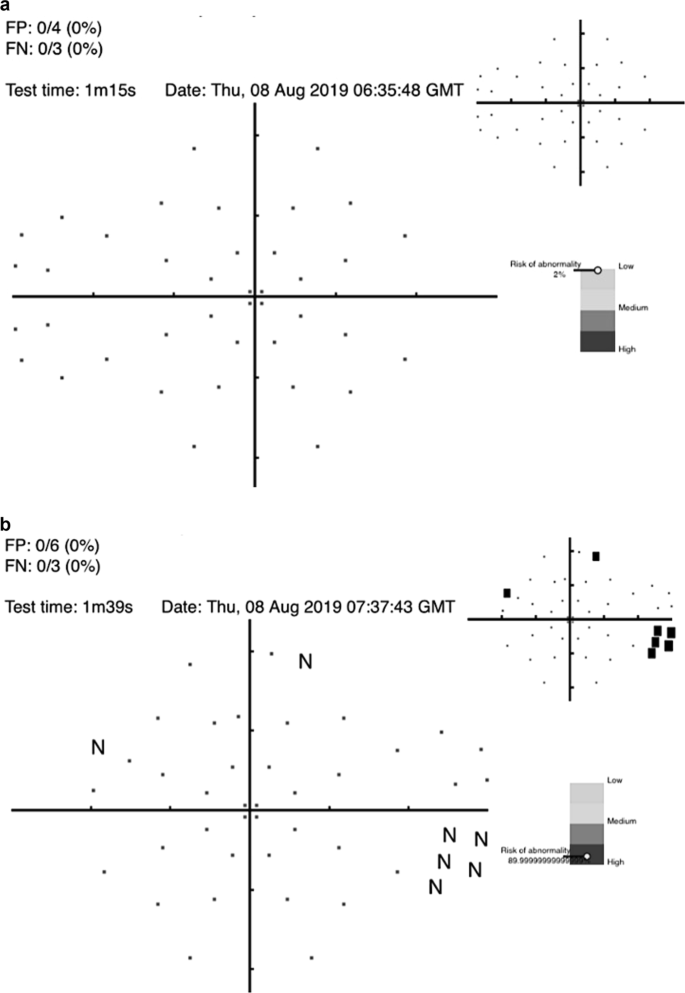
AI algorithms to diagnose glaucoma using datasets derived from VF testing have been studied since 1994 [77,78,79,80,81]. Notably, DL algorithms to diagnose glaucoma with data from standard automated perimetry (SAP) with Humphrey VF 24-2 and 30-2 SITA standard VF test outperformed the diagnostic accuracy of glaucoma experts in differentiating normal from glaucomatous VFs, with a sensitivity of 93% and specificity of 83% [82]. Furthermore, algorithms trained using a combination of OCT images and SAP VF results reached an AROC of 0.98 for identifying patients with glaucoma [83]. The cost of many dedicated testing devices is prohibitive for general application but the recent advent of a cheap screening option (the Melbourne Rapid Fields app [84]) makes this suited for such purposes. Figure 4 illustrates examples for such screening options and where the interpretation of results is given in terms of a “probability of abnormality score” (coloured bar) to assist the clinician’s decision making process and identify high risk cases.
a visual field screening result using the Melbourne Rapid Fields app [84] for a 57 year old male showing the risk for glaucoma is low. b visual field screening result using the Melbourne Rapid Fields app [84] for a 68 year old female showing the risk for glaucoma is high. The data were collected using Melbourne Rapid Fields app and required about 1−1.5 min for testing (from Chia et al. [93]).
Multi-modal AI models
Research has also utilised multimodal structural data to enhance the assessment of glaucomatous structural damage from optic disc photographs for segmentation and detection [85, 86]. A recent multimodal model was developed using the Xception model for image feature extraction and various ML algorithms such as random forest (RF), support vector machine (SVM), dense neural network (DNN), and others showed impressive area under the receiver operating characteristic curve (AUROC) values for the different algorithms: RF had an AUROC of 99.56%, SVM had 99.59%, and DNN had 99.10% while analysing the vertical cup-to-disc ratio and mean RNFL thickness in the detection of glaucoma in a population with high incidence of myopia [87]. Another recent study showed that FusionNet based on bimodal input of VF and OCT paired data demonstrated superior performance to algorithms based on VF or OCT alone [88].
Incorporation of AI products in Australian primary care
Recent advancements in imaging technologies (such as OCT and retinal photos) allow primary care clinicians and eye specialists to identify the structural damage caused by glaucoma [89]. However, these advances come with a high cost of equipment and significant time required to interpret the image or results. Moreover, it is becoming increasingly challenging for busy primary eyecare clinics to undertake such imaging without incurring substantial costs.
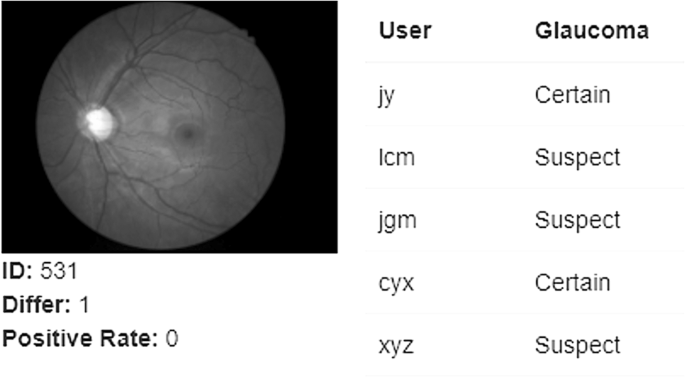
Furthermore, there is substantial variation in the visual interpretation of ONH features among clinicians [90]. This variation can be present between different clinicians (inter-observer variation) and between assessments made at different times by the same clinician (intra-observer inter-session variation). A recent study [90] involving 197 ophthalmic clinicians from 22 countries showed substantial under diagnosis based on optic nerve head photos from patients with known glaucoma. Ophthalmology trainees (22%) and comprehensive ophthalmologists (24%) consistently underestimated the likelihood of glaucoma. This level of underestimation contributes to undetected cases of glaucoma, supporting the need for AI-assisted clinical evaluation.
Automation by AI offers an opportunity to mitigate the time and cost challenges of image analysis and reduce diagnostic variation compared to human clinicians (Fig. 5). Figure 5 shows the outcome of an optic nerve image processed with AI that was consistently graded as having glaucoma by AI but returned variable diagnoses between 5 expert clinicians.
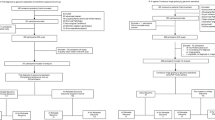
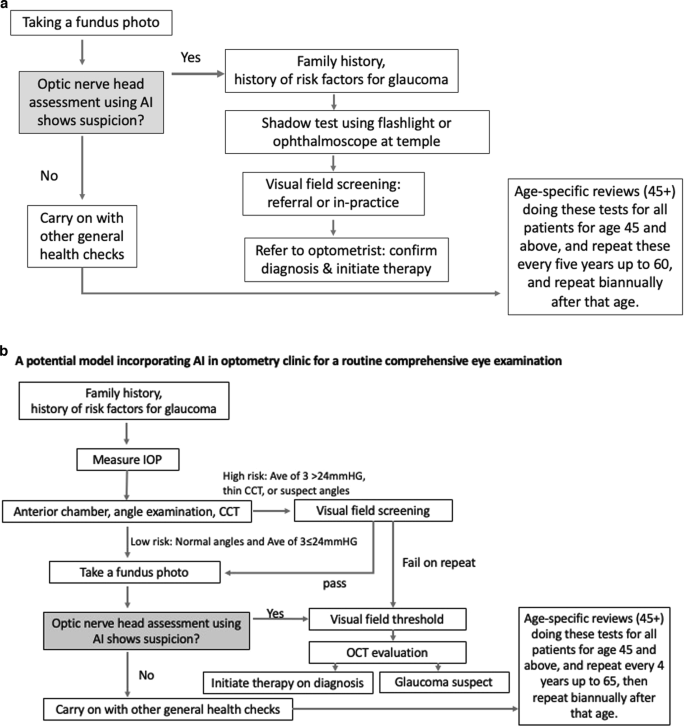
Figure 6a, b propose two potential models where AI can be incorporated into primary care settings for glaucoma detection. The assortment of tests was developed based on currently available guidelines for glaucoma detection in primary care settings [43, 91] and adopting easy to use and interpret methods for diagnosis. More research is required to investigate the best AI-assisted model for the detection of glaucoma in primary care settings.
Managing potential risks of AI use in glaucoma diagnosis
Significant progress in ML and imaging technologies enable AI to identify glaucoma signs. However, despite rapid progress in AI for glaucoma detection under laboratory settings, its real-world application has not been fully realised. The lack of a unified “gold standard” for glaucoma diagnosis is perhaps one of the biggest challenges that AI faces, as different AI algorithms focus on different aspects of the disease. For instance, the AI that focuses on evaluating the optic nerve head only evaluates structural information alone. A potential area of improvement is to develop algorithms with a more ‘holistic’ evaluation of structural and functional information, as well as information on other glaucoma risk factors such as family history and age.
Furthermore, there is a lack of prospective clinical trials applying AI in real world settings. For example, in the technologies for breast cancer screening with mammography, these issues have been particularly problematic [55]. Our team is actively trialling several AI models in optometry and GP settings and hope to publish relevant findings on the efficiency (such as accuracy, speed and acceptability of glaucoma screening) and cost-effectiveness of AI incorporation in screening programmes. In addition, real world AI implementation is impeded by challenges such as image quality, legal risks, and regulatory issues, which have barely been systematically summarised. Poor image quality can yield a high amount of ungradable images, which can lead to false positives and high health care cost. Furthermore, there have been concerns that increased reliance on AI may lead to deskilling of ophthalmic clinicians, and around privacy and cyber security as the large amount of personal information contained in the AI systems can increase the risk of data leakage. A systematic review on the patient privacy perspective on health information exchange [92] has found many patients express concerns about their health data privacy. Evidence-based protocols should be in place to safeguard algorithms and datasets against attacks. The introduction of AI-based technology may increase the cost of care, and if these costs are borne by individual patients, people of lower social economic status may remain undiagnosed. The best way for these tests to become widely available is for them to be included in health screening programmes promoted by national or private insurance companies. Our ongoing research, currently under review, pertains to cost-effectiveness analyses of AI-assisted glaucoma screening models within the context of Australia. The findings from our study indicate that the integration of AI assistance into population-based glaucoma screening programmes is cost-effective compared to traditional screening by optometrists (unpublished data, C. Jan 2023). These results suggest the economic feasibility of policy makers considering the adoption of universal glaucoma screening initiatives throughout Australia. However, it is imperative to note that the precise modalities and logistics for implementing such a programme remain to be defined. Furthermore, the medico-legal ramifications associated with placing reliance on technology for diagnostic purposes are expected to gain increasing significance and should be addressed in the context of healthcare policy and practice.
External validation studies are required to ensure the validity of deep learning algorithms and to better understand the mechanism underlying the technology or “thought process” of AI. Unlike human clinicians, current AI programmes are unable to take a holistic approach to patient care or consider other external contributing factors (such as social and psychological aspects) to management. Clinical trials are required to compare care models from practices with and without AI in real world primary care settings. AI is a tool that assists, not replaces, human clinicians.
AI-development beyond academia
Our review focuses on the prevalence of undiagnosed glaucoma in Australia and the crucial role played by primary healthcare providers in glaucoma care within the Australian healthcare system. While our primary emphasis is not on industrial advancements, it is important to acknowledge the growing prominence of AI-enabled glaucoma screening outside the academic sphere, necessitating an exploration of the latest industry developments. For instance, as of January 2023, Eyenuk (US) has achieved the noteworthy accomplishment of securing the first European Union MDR Certification for autonomous AI detection of glaucomatous optic nerve damage utilising coloured fundus photographs. Furthermore, Eyetelligence (Australia), Digital Diagnostics (US), RetinaLyze (Denmark), and Ophthalmic Sciences (Israel) are actively engaged in the development of AI-based products aimed at facilitating glaucoma screening through the analysis of fundus photographs.
Conclusion
This review highlights the significant challenge of glaucoma underdiagnosis, which can be attributed to variations in optic nerve head assessment, under-performing VF testing, and time constraints, consistent with challenges faced by primary healthcare practitioners in general in Australia. AI has shown the potential to mitigate these problems by carrying out glaucoma assessment (at least partially) in a consistent manner. The emergence of AI technology offers a promising solution to these challenges by enabling a more consistent and objective diagnosis of glaucoma. This is in contrast to the current situation, which is often characterised by inconsistent work-up and interpretation. AI algorithms have demonstrated high accuracy in diagnosing glaucoma and can provide a rapid diagnosis, thereby reducing the risk of misdiagnosis and enabling earlier treatment. Integrating AI into the diagnostic process for glaucoma has the potential to revolutionise the field and improve patient outcomes.
In primary eye care settings, advanced imaging technologies such as fundus photography and OCT, automated visual field testing, electronic health records, and large digital datasets are becoming increasingly available. These technologies can facilitate translational AI research to improve the evidence-based and consistent identification of glaucoma. As optometrists and GPs remain the first point of contact for patients with eye problems in Australia and other countries, the integration of AI into primary eye care settings has the potential to significantly improve glaucoma diagnosis and management.
Data availability
As this is a literature review, no original raw data was used.
References
Wong EY, Keeffe JE, Rait JL, Vu HT, Le A, McCarty PhD C, et al. Detection of undiagnosed glaucoma by eye health professionals. Ophthalmology. 2004;111:1508–14.
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull world health Organ. 2004;82:844–51.
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8.
Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV. et al.Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis.Lancet Glob Health. 2017;5:e1221–34.
The Department of Health. Australia Government. Visual impairment and blindness in Australia, Dec 2008. https://www.aihw.gov.au/getmedia/fc608984-1c92-48d0-b9fc-1ced9acec3ee/bulletin27.pdf.aspx?inline=true#:~:text=The%20leading%20causes%20of%20blindness,%25)%20and%20cataract%20(12%25). (accessed March 2023).
Rotchford AP, Kirwan JF, Muller MA, Johnson GJ, Roux P. Temba glaucoma study: a population-based cross-sectional survey in urban South Africa. Ophthalmology. 2003;110:376–82.
Soh Z, Yu M, Betzler BK, Majithia S, Thakur S, Tham YC, et al. The global extent of undetected glaucoma in adults: a systematic review and meta-analysis. Ophthalmology. 2021;128:1393–404.
Chua J, Baskaran M, Ong PG, Zheng Y, Wong TY, Aung T, et al. Prevalence, risk factors, and visual features of undiagnosed glaucoma: the Singapore epidemiology of eye diseases study. JAMA Ophthalmol. 2015;133:938–46.
Topouzis F, Coleman AL, Harris A, Koskosas A, Founti P, Gong G, et al. Factors associated with undiagnosed open-angle glaucoma: the Thessaloniki eye study. Am J Ophthalmol. 2008;145:327–35.e1.
Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158:1121–9.e1.
Sakata K, Sakata LM, Sakata VM, Santini C, Hopker LM, Bernardes R, et al. Prevalence of glaucoma in a South brazilian population: projeto glaucoma. Investig Ophthalmol Vis Sci. 2007;48:4974–9.
Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne visual impairment project. Ophthalmology. 1998;105:733–9.
Foreman J, Xie J, Keel S, Ang GS, Lee PY, Bourne R, et al. Prevalence and causes of unilateral vision impairment and unilateral blindness in Australia: the National Eye Health Survey. JAMA Ophthalmol. 2018;136:240–8.
Keel S, Xie J, Foreman J, Lee PY, Alwan M, Fahy ET, et al. Prevalence of glaucoma in the Australian national eye health survey. Br J Ophthalmol. 2019;103:191–5.
O'neill EC, Danesh-Meyer HV, Kong GX, Hewitt AW, Coote MA, Mackey DA, et al. Optic disc evaluation in optic neuropathies: the optic disc assessment project. Ophthalmology. 2011;118:964–70.
Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. Bmj. 2005;331:134.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311:1901–11.
Chan HH, Ong DN, Kong YX, O'Neill EC, Pandav SS, Coote MA, et al. Glaucomatous optic neuropathy evaluation (GONE) project: the effect of monoscopic versus stereoscopic viewing conditions on optic nerve evaluation. Am J Ophthalmol. 2014;157:936–44.
Weinreb RN, Leung CKS, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, et al. Primary open-angle glaucoma. Nat Rev Dis Prim Engl. 2016;2:16067.
Iyer J, Vianna JR, Chauhan BC, Quigley HA. Toward a new definition of glaucomatous optic neuropathy for clinical research. Curr Opin Ophthalmol. 2020;31:85–90.
Chauhan BC, O'leary N, AlMobarak FA, Reis A, Yang H, Sharpe GP, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120:535–43.
Jampel HD, Singh K, Lin SC, Chen TC, Francis BA, Hodapp E, et al. Assessment of visual function in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:986–1002.
Khoury JM, Donahue SP, Lavin PJ, Tsai JC. Comparison of 24-2 and 30-2 perimetry in glaucomatous and nonglaucomatous optic neuropathies. J Neuroophthalmol. 1999;19:100–8.
Yenice O, Temel A. Evaluation of two Humphrey perimetry programs: full threshold and SITA standard testing strategy for learning effect. Eur J Ophthalmol. 2005;15:209–12.
Chauhan BC, Garway-Heath DF, Goñi FJ, Rossetti L, Bengtsson B, Viswanathan AC, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–73.
Crabb DP, Garway-Heath DF. Intervals between visual field tests when monitoring the glaucomatous patient: wait-and-see approach. Investig Ophthalmol Vis Sci. 2012;53:2770–6.
Garway-Heath DF, Quartilho A, Prah P, Crabb DP, Cheng Q, Zhu H. Evaluation of visual field and imaging outcomes for glaucoma clinical trials (an American Ophthalomological Society Thesis). Trans Am Ophthalmol Soc. 2017;115:115.
The Commonwealth Fund. International Profiles of Health Care Systems. Available at https://www.commonwealthfund.org/international-health-policy-center/system-profiles. Accessed 20 Jul 2023.
Australian Institute of Health and Welfare (2023) Cancer screening programs: quarterly data, AIHW, Australian Government, https://www.aihw.gov.au/reports/cancer-screening/national-cancer-screening-programs-participation/contents/summary (Accessed 20 Jul 2023).
Optometry Australia. http://archived.optometry.org.au/blog-news/2018/8/24/medicare-a-highlight-in-100-years-of-milestones/ Accessed 27 Jan 2022.
Optometry Board of Australia. https://www.optometry.org.au/workforce/australian-optometrists-top-6000-and-two-thirds-are-therapeutically-endorsed/. Accessed 4 Feb 2022.
Optometry Australia. A decade on the PBS and nearly 100,000 scripts a year. https://www.optometry.org.au/therapeutics/a-decade-on-the-pbs-and-nearly-100000-scripts-a-year/. Accessed 25 Jul 2023
Optometry Board of Australia. Optometry Guidelines for Use of Scheduled Medicines. 2018. https://nla.gov.au/nla.obj-2996351198/view (accessed 13 March 2023).
Evidence reviews for selective laser trabeculoplasty in ocular hypertension or chronic open-angle glaucoma adult patients: Glaucoma: diagnosis and management: Evidence review A. London: National Institute for Health and Care Excellence (NICE); 2022.
Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet. 2019;393:1505–16.
Australian Government Department of Health. MBS Online: Medicare Benefits Schedule. 2020. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-202001. Accessed 26 Apr 2022.
Ford BK, Kim D, Keay L, White AJ. Glaucoma referrals from primary care and subsequent hospital management in an urban Australian hospital. Clin Exp Optom. 2020;103:821–9.
Foot B, MacEwen C. Surveillance of sight loss due to delay in ophthalmic treatment or review: frequency, cause and outcome. Eye. 2017;31:771–5.
Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12:e11686.
Kirkman JM, Bentley SA, Armitage JA, Woods CA. Could adoption of the rural pipeline concept redress Australian optometry workforce issues? Clin Exp Optom. 2019;102:566–70.
Keeffe JE, Weih LM, McCarty CA, Taylor HR. Utilisation of eye care services by urban and rural Australians. Br J Ophthalmol. 2002;86:24–7.
Burr J, Mowatt G, Hernández R, Siddiqui M, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–190.
US Preventive Services Task F, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, et al. Screening for primary open-angle glaucoma: US preventive services task force recommendation statement. JAMA. 2022;327:1992–7.
Toomey M, Ho KC, Gyawali R, Stapleton F, Wiles L, Hibbert P, et al. The appropriateness of and barriers to glaucoma care delivery by Australian optometrists. Clin Exp Optom. 2022;105:1–9.
Elam AR, Andrews C, Musch DC, Lee PP, Stein JD. Large disparities in receipt of glaucoma care between enrollees in Medicaid and those with commercial health insurance. Ophthalmology. 2017;124:1442–8.
Stein JD, Talwar N, LaVerne AM, Nan B, Lichter PR. Racial disparities in the use of ancillary testing to evaluate individuals with open-angle glaucoma. Arch Ophthalmol. 2012;130:1579–88.
Deep Blue https://www.ibm.com/ibm/history/ibm100/us/en/icons/deepblue/. Accessed 20 Sept 2023.
Tromp J, Farnebäck G. Combinatorics of go. International Conference on Computers and Games. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 84–99.
Lee KF. AI superpowers: China, Silicon Valley, and the new world order. Houghton Mifflin; 2018.
Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, et al. Mastering the game of Go without human knowledge. Nature. 2017;550:354–9.
Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE-an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141:1032–4.
Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197:11–7.
Wolf JA, Moreau JF, Akilov O, Patton T, English JC, Ho J, et al. Diagnostic inaccuracy of smartphone applications for melanoma detection. JAMA Dermatol. 2013;149:422–6.
Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig Ophthalmol Vis Sci. 2016;57:5200–6.
Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. Jama. 2017;318:2199–210.
Manak MS, Varsanik JS, Hogan BJ, Whitfield MJ, Su WR, Joshi N, et al. Live-cell phenotypic-biomarker microfluidic assay for the risk stratification of cancer patients via machine learning. Nat Biomed Eng. 2018;2:761–72.
Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284:574–82.
FDA permits marketing of AI software that autonomously detects diabetic retinopathy. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye (Accessed 10 July 2023).
Fan Z, Rong Y, Cai X, Lu J, Li W, Lin H, et al. Optic disk detection in fundus image based on structured learning. IEEE J Biomed Health Inf. 2018;22:224–34.
Mookiah MR, Acharya UR, Chua CK, Min LC, Ng EY, Mushrif MM, et al. Automated detection of optic disk in retinal fundus images using intuitionistic fuzzy histon segmentation. Proc Inst Mech Eng H J Eng Med. 2013;227:37–49.
Liu H, Li L, Wormstone IM, Qiao C, Zhang C, Liu P, et al. Development and validation of a deep learning system to detect glaucomatous optic neuropathy using fundus photographs. JAMA Ophthalmol. 2019;137:1353–60.
Medeiros FA, Jammal AA, Thompson AC. From machine to machine: an OCT-trained deep learning algorithm for objective quantification of glaucomatous damage in fundus photographs. Ophthalmology. 2019;126:513–21.
Thompson AC, Jammal AA, Medeiros FA. A deep learning algorithm to quantify neuroretinal rim loss from optic disc photographs. Am J Ophthalmol. 2019;201:9–18.
Jammal AA, Thompson AC, Mariottoni EB, Berchuck SI, Urata CN, Estrela T, et al. Human versus machine: comparing a deep learning algorithm to human gradings for detecting glaucoma on fundus photographs. Am J Ophthalmol. 2020;211:123–31.
Raghavendra U, Fujita H, Bhandary SV, Gudigar A, Tan JH, Acharya UR. Deep convolution neural network for accurate diagnosis of glaucoma using digital fundus images. Inf Sci. 2018;441:41–9.
Li Z, He Y, Keel S, Meng W, Chang RT, He M. Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology. 2018;125:1199–206.
Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98:ii15–9.
Huang ML, Chen HY. Development and comparison of automated classifiers for glaucoma diagnosis using Stratus optical coherence tomography. Investig Ophthalmol Vis Sci. 2005;46:4121–9.
Naithani P, Sihota R, Sony P, Dada T, Gupta V, Kondal D, et al. Evaluation of optical coherence tomography and heidelberg retinal tomography parameters in detecting early and moderate glaucoma. Investig Ophthalmol Vis Sci. 2007;48:3138–45.
Barella KA, Costa VP, Gonçalves Vidotti V, Silva FR, Dias M, Gomi ES. Glaucoma diagnostic accuracy of machine learning classifiers using retinal nerve fiber layer and optic nerve data from SD-OCT. J Ophthalmol. 2013;2013:789129.
Bizios D, Heijl A, Hougaard JL, Bengtsson B. Machine learning classifiers for glaucoma diagnosis based on classification of retinal nerve fibre layer thickness parameters measured by Stratus OCT. Acta Ophthalmol. 2010;88:44–52.
Larrosa JM, Polo V, Ferreras A, García-Martín E, Calvo P, Pablo LE. Neural network analysis of different segmentation strategies of nerve fiber layer assessment for glaucoma diagnosis. J Glaucoma. 2015;24:672–8.
Muhammad H, Fuchs TJ, De Cuir N, De Moraes CG, Blumberg DM, Liebmann JM, et al. Hybrid deep learning on single wide-field optical coherence tomography scans accurately classifies glaucoma suspects. J Glaucoma. 2017;26:1086–94.
Xu J, Ishikawa H, Wollstein G, Bilonick RA, Folio LS, Nadler Z, et al. Three-dimensional spectral-domain optical coherence tomography data analysis for glaucoma detection. PLoS One. 2013;8:e55476.
Burgansky-Eliash Z, Wollstein G, Chu T, Ramsey JD, Glymour C, Noecker RJ, et al. Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest Ophthalmol Vis Sci. 2005;46:4147–52.
Maetschke S, Antony B, Ishikawa H, Wollstein G, Schuman J, Garnavi R. A feature agnostic approach for glaucoma detection in OCT volumes. PLoS One. 2019;14:e0219126.
Andersson S, Heijl A, Bizios D, Bengtsson B. Comparison of clinicians and an artificial neural network regarding accuracy and certainty in performance of visual field assessment for the diagnosis of glaucoma. Acta Ophthalmol. 2013;91:413–7.
Asaoka R, Murata H, Iwase A, Araie M. Detecting preperimetric glaucoma with standard automated perimetry using a deep learning classifier. Ophthalmology. 2016;123:1974–80.
Bowd C, Weinreb RN, Balasubramanian M, Lee I, Jang G, Yousefi S, et al. Glaucomatous patterns in Frequency Doubling Technology (FDT) perimetry data identified by unsupervised machine learning classifiers. PLoS One. 2014;9:e85941.
Cai S, Elze T, Bex PJ, Wiggs JL, Pasquale LR, Shen LQ. Clinical correlates of computationally derived visual field defect archetypes in patients from a glaucoma clinic. Curr Eye Res. 2017;42:568–74.
Goldbaum MH, Sample PA, White H, Côlt B, Raphaelian P, Fechtner RD, et al. Interpretation of automated perimetry for glaucoma by neural network. Invest Ophthalmol Vis Sci. 1994;35:3362–73.
Li F, Wang Z, Qu G, Song D, Yuan Y, Xu Y, et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med Imaging. 2018;18:35.
Bizios D, Heijl A, Bengtsson B. Integration and fusion of standard automated perimetry and optical coherence tomography data for improved automated glaucoma diagnostics. BMC Ophthalmol. 2011;11:20.
What is MRF Visual Field Test? https://www.appviewmrf.com/what-is-mrf-visual-field-test/. Accessed 20 Sept 2023.
Wu M, Leng T, de Sisternes L, Rubin DL, Chen Q. Automated segmentation of optic disc in SD-OCT images and cup-to-disc ratios quantification by patch searching-based neural canal opening detection. Opt Express. 2015;23:31216–29.
Babu T, Devi S, Venkatesh R. Optic nerve head segmentation using fundus images and optical coherence tomography images for glaucoma detection. Biomed Pap. 2015;159:607–15.
Lim WS, Ho H-Y, Ho H-C, Chen YW, Lee CK, Chen PJ, et al. Use of multimodal dataset in AI for detecting glaucoma based on fundus photographs assessed with OCT: focus group study on high prevalence of myopia. BMC Med Imaging. 2022;22:1–14.
Xiong J, Li F, Song D, Tang G, He J, Gao K, et al. Multimodal machine learning using visual fields and peripapillary circular OCT scans in detection of glaucomatous optic neuropathy. Ophthalmology. 2022;129:171–80.
Dick HB, Schultz T, Gerste RD. Miniaturization in glaucoma monitoring and treatment: a review of new technologies that require a minimal surgical approach. Ophthalmol Ther. 2019;8:19–30.
O'neill EC, Gurria LU, Pandav SS, Kong YX, Brennan JF, Xie J, et al. Glaucomatous optic neuropathy evaluation project: factors associated with underestimation of glaucoma likelihood. JAMA Ophthalmol. 2014;132:560–6.
Clinical Practice Guide for the Diagnosis and Management of Open Angle Glaucoma 2020. https://www.optometry.org.au/wp-content/uploads/Professional_support/Guidelines/Glaucoma-Clinical-Practice-Guide_Dec-2020_design_v6.pdf (Accessed 13 March 2023).
Shen N, Bernier T, Sequeira L, Strauss J, Silver MP, Carter-Langford A, et al. Understanding the patient privacy perspective on health information exchange: a systematic review. Int J Med Inform. 2019;125:1–12.
Chia MA, Trang E, Agar A, Vingrys AJ, Hepschke J, Kong GY, et al. Screening for glaucomatous visual field defects in rural Australia with an iPad. J Curr Glaucoma Pract. 2021;15:125–31.
Russell, SJ & Norvig, P. Artificial Intelligence: A Modern Approach (Prentice Hall, New Jersey, 2021).
Murphy, KP & Bach F Machine Learning: A Probabilistic Perspective (MIT Press, Cambridge, 2012).
Goodfellow, I, Bengio, Y, Courville, A & Bengio, Y Deep Learning (MIT Press, Cambridge, 2016).
Acknowledgements
This project received grant funding from the Australian Government: the National Critical Research Infrastructure Initiative, Medical Research Future Fund (MRFAI00035) and the NHMRC Investigator Grant (APP1175405). The contents of the published material are solely the responsibility of the Administering Institution, a participating institution or individual authors and do not reflect the views of the NHMRC. The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government. CJ is supported by Research Training Scholarship from the Australian Commonwealth Government. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
CJ was responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analysing data, interpreting results, and updating reference lists. MH was responsible for interpreting results and providing critical review of the report. AV was responsible for designing the review protocol, interpreting results, providing additional references, and providing critical review of the report. ZZ was responsible for interpreting results and providing critical review of the report. RSS was responsible for interpreting results and providing critical review of the report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jan, C., He, M., Vingrys, A. et al. Diagnosing glaucoma in primary eye care and the role of Artificial Intelligence applications for reducing the prevalence of undetected glaucoma in Australia. Eye (2024). https://doi.org/10.1038/s41433-024-03026-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-024-03026-z