Abstract
Background
Idiopathic full-thickness macular hole (iFTMH) closure rates following conventional vitrectomy, gas tamponade and internal limiting membrane (ILM) peeling decrease when the minimum linear diameter (MLD) ≥ 500 microns. ILM flap creation has been proposed to improve closure in larger holes. This study evaluated the anatomical and functional impact of ILM flap introduction to routine practice in iFTMH ≥500 microns.
Methods
Retrospective, interventional analysis of prospectively collected data of 191 eyes from consecutive surgeries for primary iFTMH ≥500 microns performed by two surgeons between June 2018 and June 2022, during which both surgeons replaced ILM peeling with ILM flap creation. Post-operative best-corrected visual acuity (BCVA) and anatomical closure were compared between Group 1 (ILM peel) and Group 2 (ILM flap) in an intention-to-treat analysis.
Results
Rates of iFTMH closure were greater in the ILM flap group (77/80; 96.3%) than the ILM peel group (94/110; 85.5%) (OR = 4.37, 95% CI = 1.23–15.55, p = 0.023). A non-significant increase in post-operative BCVA improvement was observed in the ILM flap group (p = 0.084). There was no statistically significant difference in final BCVA (p = 0.83). Multivariate logistic regression found only MLD (OR = 0.993, 95% CI = 0.989–0.997, p = 0.001) and ILM flap group (OR = 5.795, 95% CI = 1.313–25.570, p = 0.020) predicted primary closure.
Conclusion
ILM flap creation improves closure rates in larger holes and should be considered routinely in iFTMH ≥500 microns. Whether ILM flaps affect post-operative visual function remains uncertain.
Similar content being viewed by others

Introduction
Idiopathic full-thickness macular holes (iFTMH) are full thickness foveal defects [1]. They typically result in significant visual impairment and have an overall incidence of approximately 3–8 per 100,000 per annum, rising to 33 per 100,000 in women in their 60–70 s [2,3,4,5]. Since the introduction of internal limiting membrane (ILM) peeling in 1996 [6], the success rate of iFTMH anatomical closure following conventional surgery involving vitrectomy, ILM peeling and gas tamponade has climbed to around 96% overall in consecutive case series [7].
Macular hole size however is known to be an important factor in predicting surgical success, with closure reducing as size increases [8]. Whilst a minimum linear diameter (MLD) of 400 microns has been used as a cut-off for large full thickness macular holes [1], it has been shown that surgical success rate only starts to decline when macular hole width exceeds around 500 microns in size, to 90% or less in a large UK database study [7]. This was corroborated in another UK single centre series, which documented a further decline in closure above 630 microns [9]. Surgeons have therefore explored several surgical variations and adjuncts in these larger macular holes to improve success rates.
Michalewska et al. [10] first presented a novel technique of inverted ILM flap for the treatment of large macular holes, which appeared to improve closure rates. They hypothesised ILM flaps may stimulate gliosis and act as a scaffold for tissue proliferation. This was later supported by an experimental primate model which found activated Müller cells on the ILM flap producing neurotrophic factors that may contribute to macular hole closure [11]. Recently, randomised controlled studies (RCTs) have supported the benefit of ILM flaps in terms of closure in large holes [12, 13], whilst others have no found no conclusive benefit [14, 15]. A systematic review and meta-analysis has also shown a statistically significant improved closure rate of large macular holes with the use of ILM flaps compared to ILM peeling with an odds ratio of 3.95, but no difference in long-term visual outcomes [16]. The effect on visual acuity is unclear, although a large recent retrospective study has suggested a benefit [17].
There has been limited real-world data published on the routine use of ILM flaps in large macular holes over 500 microns, including those with variable symptom duration, which is also known to affect closure and visual outcomes, and which is typically constrained in randomised controlled trials [18]. The aim of this study was to evaluate the real-world anatomical and functional outcomes by comparing conventional ILM peeling to the use of ILM flaps in treating iFTMH of 500 microns or larger.
Subjects and methods
We retrospectively reviewed all consecutive surgeries for primary iFTMH of 500 microns or larger, performed by two consultant vitreoretinal surgeons in two UK surgical units (DS in Sunderland Eye Infirmary and DY in Gartnavel Hospital, Glasgow). The patients were identified using prospectively collected data from the BEAVRS vitreoretinal database with a data collection period from June 2018 to June 2022. DS started using ILM flaps routinely in these cases from March 2019 and DY from October 2019.
Both surgeons used the same technique of ILM flaps, namely a superior single layer flap [19]. Prior to these dates all macular holes had been treated with vitrectomy, conventional ILM peeling with a peel radius of 1–2-disc diameters around the hole centre and predominantly long-acting gas tamponade (16–18% C2F6). Post-operatively, all patients were asked to posture face down for 1 day and avoid supine positioning for 1 week. Combined phacovitrectomy was performed in all phakic patients. Throughout the study period the surgeons’ techniques did not change in any other respects.
We excluded cases of vitrectomy performed with silicone oil tamponade, previous vitrectomised eyes, non-full thickness macular holes, persistent FTMH undergoing further re-operation and secondary macular holes including those which have developed in association with trauma, retinal detachment, myopia >6 dioptres or retinal dystrophies. Operations with less than 8 weeks follow-up were also not included in any post-operative best-corrected visual acuity (BCVA) analysis.
All iFTMH were imaged immediately preoperatively using spectral domain optical coherence tomography (SDOCT). The MLD was determined on the horizontal line scan where the hole was widest and measured manually using built-in-calliper tool [20]. Post-operatively, closure was assessed using SDOCT and specified as complete neurosensory retinal closure in any configuration without a retinal defect.
We collected baseline characteristics on age, sex, symptom duration, lens status, ocular co-morbidities, pre- and post-operative BCVA, pre-operative MLD, types of gas tamponade used and post-operative anatomical success. Primary outcome measures were post-operative BCVA; and successful anatomical closure following a single surgery.
We present descriptive data using tabular and graphical summaries. Statistical analyses were performed using MedCalc (MedCalc® Statistical Software version 20.218 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2023). Quantitative variables were presented as frequencies with percentages. Non-parametric continuous variables were reported as median, IQR, and range. Differences in median values were assessed with Mann-Whitney tests. We expressed results using odds ratios (ORs) and their 95% confidence intervals (CIs). Multivariate analysis by stepwise logistic regression was used to examine variables associated with primary closure of iFTMH.
We included all consecutive eyes in the analyses dependant on their follow-up duration. Eyes had to have had at least 2 weeks post-operative follow up to be included in the anatomical analysis and 8 weeks to be included in the visual outcome analysis, Supplementary Fig. 1. We included all eyes with attempted ILM flap creation in an intention-to-treat (ITT) analysis.
Results
191 consecutive eyes with holes >500 microns, operated on by the two surgeons within the specified time period were identified from the BEAVRS database, with 111 eyes undergoing ILM peeling in the first time period (ILM peel group) and 80 eyes undergoing ILM flap in the second time period (ILM flap group). Two ILM flaps were avulsed in group 2 but were included in the intention-to treat analysis.
The baseline characteristics of the two treatment cohorts are shown in Table 1. No statistically significant differences were observed in the age, sex, MLD, BD, baseline phakic status, gas tamponade used, or duration of follow-up between the two groups. The symptom duration prior to surgery was significantly greater in the ILM flap group (median 10 months) than the ILM peeling group (median 7 months, p = 0.001), whilst the pre-operative BCVA was also worse in the ILM flap group than the ILM peeling group (1.1 to 1.0 logMAR, p = 0.015).
The ILM peel and the ILM flap groups had 110 (99.1%) and 80 (100%) eyes eligible for anatomical closure assessment with follow-up >2 weeks post-op, respectively, whilst 107 (96.4%) and 72 (90%) eyes of the ILM peel and ILM flap group respectively were eligible for visual function assessment, with acuity data at >8 weeks post-op.
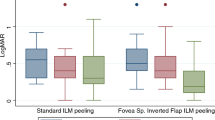
Post-operative outcomes are shown in Table 2. FTMH closure was more likely in the ILM flap group, with 77 (96.3%) in the ILM flap group closing, compared to 94 (85.5%) of the ILM peel group (OR = 4.37, p = 0.023). Of the 19 eyes without primary closure, all but 1 had revision surgery with 15 of those 18 achieving closure. The final closure rate was therefore 186 of the 190 (97.9%) eyes in total. An increase in post-operative BCVA improvement was observed in the ILM flap group (median 0.60) compared to the ILM peel cohort (median 0.52, p = 0.084). There was no difference in final BCVA, or in the proportion gaining or losing ≥0.2 logMAR between the two cohorts. The results were unchanged when considering only those with primary closure.
Multivariate logistic regression showed that only two factors predicted primary closure, namely MLD and being in the ILM flap group, Table 3.
Discussion
Using prospectively collected real-world data from two surgeons before and after the routine use of ILM flaps in iFTMH > 500 microns in size, we show that the introduction of ILM flaps improved the closure rate with both clinical and statistical significance. The closure rate increased from 86 to 96%. Multivariate analysis showed that ILM flap use had a beneficial effect on closure with an OR of 5.8.
As mentioned in the introduction, recently published real-world based studies have proposed that the definition of a large FTMH, when the MLD is >400 microns, is not a pragmatic demarcation point in terms of surgical success [1, 7, 10, 21]. 500 microns may be a more appropriate threshold where the success rate drops from above 95%, to below 90%. We observed closure rates in FTMH > 500 microns improving from 86 to 96% following the introduction of ILM flaps. This closure rate is similar to that observed in smaller holes, following conventional surgery [7, 9].
Our study corroborates previous retrospective, non-randomised studies investigating the impact of ILM flap on closure rates in large FTMH: Baumann et al. [22]. observed a similar closure rate in their study of 117 eyes with an MLD > 400 microns, with 99% achieving successful closure in the ILM flap group compared to 88% in ILM peel group. Rizzo et al. [17]. in their comparative study of over 620 eyes also observed a significant improvement in closure rates from 79 to 92% in all holes, whilst specifically in holes >400 microns, an increase from 79% closure with ILM peeling to 96% with ILM flaps was observed. A meta-analysis of 4 RCTs and 4 retrospective studies conducted by Shen et al. [16]. also reported superior closure rates with ILM flaps, although they all included holes >400 microns rather than 500 microns. Our unadjusted OR of 4.4 concurs with their pooled OR of 4, whilst our multivariate analysis suggests that in larger holes (>500 microns) the effect is greater still.
Despite the higher closure rate with ILM flaps, similar post-operative visual function was observed in both groups. The change from ILM peel to ILM flap was instituted by both surgeons just before the coronavirus (COVID-19) pandemic, and the ILM flap group therefore included patients with significantly longer symptom durations and worse baseline visual acuities than the ILM peel group as other procedures were prioritised in the UK during phases of the pandemic [23]. Both variables are established negative predictors of post-operative visual function and indeed closure, potentially confounding any beneficial effect on post-operative visual function [16]. Furthermore, all patients with recalcitrant holes in our study underwent repeat surgery with high closure rates, further reducing the difference in acuity at their last visit. Despite these observations it is worth noting that we showed greater improvement in visual acuity in the ILM flap group, although there is a possibility that this difference occurred by chance.
Whether ILM flaps improve post-operative visual function is disputed in the current literature. Of the 8 RCTs published to date comparing ILM flap use to conventional ILM peeling (Table 4), only 3 report significantly improved post-operative visual function in the ILM flap group, albeit these RCTs were conducted in large holes and had the largest sample sizes [10, 12, 13]. Of the 4 RCTs demonstrating no significant improvement in post-operative visual function, 3 were conducted in small-medium holes, whilst the other on larger holes which did not reach significance had a smaller sample size than the other large hole RCTs [14, 21, 24, 25]. Furthermore, Ventre et al. [21]. in their RCT of small-medium holes further demonstrated worse final post-operative macular sensitivity following ILM flap than ILM peel. There is also contradictory evidence from published retrospective studies: Rizzo et al. [17] observed an improvement in post-operative visual function in all sized holes, including those >400 microns, whilst Baumann et al. [22] and Yamashita et al. [26] did not observe a difference in holes >400 microns. The data to date suggests that the main benefit of ILM flaps is observed in patients with larger holes, where the risk benefit ratio of the more prolonged surgery required to create a flap is clearer.
Recent evidence published by Chou et al. [27] in FTMH < 400 microns treated with ILM flaps shows significantly improved post-op BCVA at 1, 3 and 6 months, but not at 12 months. This is in keeping with the findings of Kwak et al. [28] who observed superior post-operative BCVA at 1 month post-op, but not at 3-, 6- or 12 months, where propensity-score matching was used to eliminate the influence of important baseline confounders such as MLD and pre-operative BCVA. The meta-analysis by Shen et al. [16] also reported an improved post-operative BCVA at 3-months, but not 6 months. We did not analyse post-operative visual function at multiple time intervals and required at least 8 weeks follow-up for visual outcome analysis, therefore we are unable to compare our findings to the short-term improvements in visual function reported. Notably, in our study the follow-up in the two groups was comparable. It is possible that the early improvement in visual function noted by these authors arises from the superior initial closure rates, and at later time points after revision surgery in non-closed holes the effect diminishes. It has also been hypothesised that ILM flaps may mechanically obstruct the restoration of the ellipsoid zone and external limiting membrane, essential to good post-operative visual function in FTMH repair [29,30,31,32]. Future studies with larger cohorts and long follow-up of at least 12 months are required to evaluate whether the initial impact of increased primary closure is accompanied by any beneficial or detrimental effect on post-operative visual function.
Since the conception of the ILM flap technique by Michalewska et al. [10], multiple variations have been proposed which can be broadly divided into single layer covering techniques and flap insertion techniques as originally described. There is no definitive evidence published that one technique is superior to the other, although one study suggests single layer techniques have improved visual results in the short term [33]. In our study, both surgeons used the large superior semicircular inverted ILM flap technique described by Chen et al. [19], a variation of the covering single layer flap, and therefore we avoided any potential negative effects of the insertion technique.
Unlike randomised trials, our patients were consecutive cases, without exclusions for prolonged duration or co-morbidity. These results are likely to be applicable to the real-world experience of retinal surgeons. Our study is however not without limitations, notably a lack of randomisation meaning that we cannot discount unrecognised confounding effects, although this was mitigated as our groups were consecutive cases, and with prospective data collection. Both surgeons had over 15 years of experience of ILM peeling for FTMH prior to the study start date making experiential effects unlikely. Similarly, the surgery was standardised between both groups including for post-operative positioning. Both surgeons only asked patients to posture for 24 h whilst they were inpatients, making any confounding effect of posturing also improbable. Our population was predominantly White British, with a relatively long symptom duration limiting generalisability in different populations. Despite these limitations, we feel the results from our study can be translated to routine clinical practice for all FTMH > 500 microns as our data come from the consecutive cases of two surgeons and analysed as an ITT analysis accepting that 2 ILM flaps avulsed during creation, acknowledging the technical challenges of performing the procedure. Finally, we did not describe the exact closure pattern, but it should be noted that our definition of closure was type 1 without a neurosensory defect, and type 2 closures were classed a non-closure.
In conclusion in this consecutive real world by two independent surgeons case series we have demonstrated that the use of ILM flaps in large FTMH > 500 microns improves closure rates. Whether ILM flaps effect post-operative visual function remains unclear and requires further study. The use of ILM flaps should be considered routinely in patients with iFTMH and a MLD of greater than 500 microns.
Summary
What was known before
-
The use of ILM flaps probably improves FTMH closure in holes greater than 400 microns.
What this study adds
-
Robust, generalisable, real-world evidence that the use of ILM flaps as part of the routine surgical management of FTMH > 500 microns will substantially increase the likelihood of closure and reduce the need for re-operation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–9.
Cho SC, Park SJ, Byun SJ, Woo SJ, Park KH. Five-year nationwide incidence of macular hole requiring surgery in Korea. Br J Ophthalmol. 2019;103:1619–23.
Darian-Smith E, Howie AR, Allen PL, Vote BJ. Tasmanian macular hole study: whole population-based incidence of full thickness macular hole. Clin Exp Ophthalmol. 2016;44:812–6.
McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology. 2009;116:1366–9.
Forsaa VA, Lindtjørn B, Kvaløy JT, Frøystein T, Krohn J. Epidemiology and morphology of full-thickness macular holes. Acta Ophthalmol. 2018;96:397–404.
Eckardt C, Eckardt U, Groos S, Luciano L, Reale E. [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings]. Ophthalmologe. 1997;94:545–51.
Steel DH, Donachie PHJ, Aylward GW, Laidlaw DA, Williamson TH, Yorston D. Factors affecting anatomical and visual outcome after macular hole surgery: findings from a large prospective UK cohort. Eye. 2021;35:316–25.
Ullrich S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390–3.
Ch’ng SW, Patton N, Ahmed M, Ivanova T, Baumann C, Charles S, et al. The manchester large macular hole study: is it time to reclassify large macular holes? Am J Ophthalmol. 2018;195:36–42.
Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–25.
Shiode Y, Morizane Y, Matoba R, Hirano M, Doi S, Toshima S, et al. The role of inverted internal limiting membrane flap in macular hole closure. Invest Ophthalmol Vis Sci. 2017;58:4847–55.
Agrawal V, Jindal K, Dhakad Y, Rathore P, Khilnani K. Multilayered inverted internal limiting membrane flap technique versus standard internal limiting membrane peeling for large macular holes: a comparative study. Indian J Ophthalmol. 2022;70:909–13.
Manasa S, Kakkar P, Kumar A, Chandra P, Kumar V, Ravani R. Comparative evaluation of standard ILM peel with inverted ILM flap technique in large macular holes: a prospective, randomized study. Ophthalmic Surg Lasers Imaging Retina. 2018;49:236–40.
Kannan NB, Kohli P, Parida H, Adenuga OO, Ramasamy K. Comparative study of inverted internal limiting membrane (ILM) flap and ILM peeling technique in large macular holes: a randomized-control trial. BMC Ophthalmol. 2018;18:177.
Velez-Montoya R, Ramirez-Estudillo JA, Sjoholm-Gomez de Liano C, Bejar-Cornejo F, Sanchez-Ramos J, Guerrero-Naranjo JL, et al. Inverted ILM flap, free ILM flap and conventional ILM peeling for large macular holes. Int J Retin Vitreous. 2018;4:8.
Shen Y, Lin X, Zhang L, Wu M. Comparative efficacy evaluation of inverted internal limiting membrane flap technique and internal limiting membrane peeling in large macular holes: a systematic review and meta-analysis. BMC Ophthalmol. 2020;20:14.
Rizzo S, Tartaro R, Barca F, Caporossi T, Bacherini D, Giansanti F. Internal limiting membrane peeling versus inverted flap technique for treatment of full-thickness macular holes: a comparative study in a large series of patients. Retina. 2018;38:S73–s8.
Murphy DC, Al-Zubaidy M, Lois N, Scott N, Steel DH. The effect of macular hole duration on surgical outcomes: an individual participant data study of randomized controlled trials. Ophthalmology. 2023;130:152–63.
Chen SN. Large semicircular inverted internal limiting membrane flap in the treatment of macular hole in high myopia. Graefes Arch Clin Exp Ophthalmol. 2017;255:2337–45.
Steel DH, Downey L, Greiner K, Heimann H, Jackson TL, Koshy Z, et al. The design and validation of an optical coherence tomography-based classification system for focal vitreomacular traction. Eye. 2016;30:314–24.
Ventre L, Fallico M, Longo A, Parisi G, Russo A, Bonfiglio V, et al. Conventional internal limiting membrane peeling versus inverted flap for small-to-medium idiopathic macular hole: a randomized trial. Retina. 2022;42:2251–7.
Baumann C, Kaye S, Iannetta D, Sultan Z, Dwivedi R, Pearce I. Effect of inverted internal limiting membrane flap on closure rate, postoperative visual acuity, and restoration of outer retinal layers in primary idiopathic macular hole surgery. Retina. 2020;40:1955–63.
The Royal College of Ophthalmologists. Guidance document: Prioritisation of ophthalmic procedures: The Royal College of Ophthalmologists. 2020. https://www.rcophth.ac.uk/resources-listing/prioritisation-of-ophthalmic-procedures/.
Leisser C, Ruiss M, Pilwachs C, Findl O. ILM peeling with ILM flap transposition vs. classic ILM peeling for small and medium macula holes-a prospective randomized trial. Spektrum Augenheilkd. 2023;37:9–14.
Ehrhardt A, Delpuech M, Luc A, Zessler A, Pastor G, Angioi-Duprez K, et al. Dissociated optic nerve fiber layer appearance after macular hole surgery: a randomized controlled trial comparing the temporal inverted internal limiting membrane flap technique with conventional peeling. Ophthalmol Retina. 2023;7:227–35.
Yamashita T, Sakamoto T, Terasaki H, Iwasaki M, Ogushi Y, Okamoto F, et al. Best surgical technique and outcomes for large macular holes: retrospective multicentre study in Japan. Acta Ophthalmol. 2018;96:e904–e10.
Chou HD, Liu L, Wang CT, Chen KJ, Wu WC, Hwang YS, et al. Single-layer inverted internal limiting membrane flap versus conventional peel for small- or medium-sized full-thickness macular holes. Am J Ophthalmol. 2022;235:111–9.
Kwak JJ, Byeon SH. Comparison of long-term visual and anatomical outcomes between internal limiting membrane flap and peeling techniques for macular holes with a propensity score analysis. Eye. 2022;37:1207–13.
Baba T, Yamamoto S, Arai M, Arai E, Sugawara T, Mitamura Y, et al. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina. 2008;28:453–8.
Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–24.
Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Significant correlation between visual acuity and recovery of foveal cone microstructures after macular hole surgery. Am J Ophthalmol. 2012;153:111–9.
Bleidißel N, Friedrich J, Feucht N, Klaas J, Maier M. Visual improvement and regeneration of retinal layers in eyes with small, medium, and large idiopathic full-thickness macular holes treated with the inverted internal limiting membrane flap technique over a period of 12 months. Graefes Arch Clin Exp Ophthalmol. 2022;260:3161–71.
Rossi T, Gelso A, Costagliola C, Trillo C, Costa A, Gesualdo C, et al. Macular hole closure patterns associated with different internal limiting membrane flap techniques. Graefes Arch Clin Exp Ophthalmol. 2017;255:1073–8.
Author information
Authors and Affiliations
Contributions
DS and DY designed the study. DS and DY collected the data. DY analysed the data. All authors interpreted the data. GR, BLT and DS drafted the paper. All authors substantively revised the paper.
Corresponding author
Ethics declarations
Competing interests
DS declares that his institutions have received grant funding for studies from Alcon, DORC, Bayer, Boehringer and Gyroscope, and consultancy fees from BVI, Alcon, Gyroscope and Roche all unrelated to this work. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riding, G., Teh, B.L., Yorston, D. et al. Comparison of the use of internal limiting membrane flaps versus conventional ILM peeling on post-operative anatomical and visual outcomes in large macular holes. Eye (2024). https://doi.org/10.1038/s41433-024-03024-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-024-03024-1