Abstract
Background/Objectives
Uveitis in children and young people (CYP) is a rare but potentially debilitating condition. Steroid eye drops are the first step in treatment and poor compliance may result in vision-threatening complications. This study aims to measure compliance with prescribed eye drops prospectively in a child-specific manner.
Subjects/Methods
Patients aged 0–18 years attending a tertiary paediatric uveitis clinic using steroid drops were recruited. Both the CYP, and person with parental responsibility (PPR) completed questionnaires about compliance. A subgroup had bottles of Prednisolone 1% drops dispensed and weighed at the first appointment and reweighed at follow-up. The weight reduction was compared with expected weight change over the interval.
Results
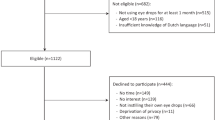
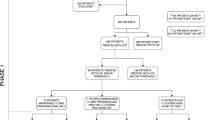
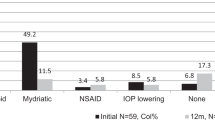
The study was completed by 42 patients of the 50 patients recruited. Thirty-one CYP and their respective PPR completed both questionnaires, 11 completed only one questionnaire (9 CYP, 2 PPR). Drop errors for all eye drops were reported more than “once a week” by 13/39 CYP (33.3%, 95% CI: 19.1%–50.2% of respondents), and 3/31 PPR (9.7%, CI: 19.1%–50.2% of respondents). Many PPR could not recall prescribed drop frequency (n = 13/31, 40.6%, CI: 23.7%–59.4% of respondents). Twelve patients had bottles weighed and returned. Insufficient weight reduction was found in 9 (75%, CI: 42.8%–94.5%). Within the eye drop weighing subgroup three participants (25%, CI: 5.5%–57.2%) used <50% the expected weight of drops.
Conclusions
This study demonstrated poor eye drop compliance in CYP with uveitis. Self-reported compliance was unreliable in this population. Worryingly, some patients miss more than 50% of drops and may suffer sub-optimal disease control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The dataset from the current study is available from the corresponding author on reasonable request.
References
Gregory AC, Kempen JH, Daniel E, Kaçmaz RO, Foster CS, Jabs DA, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the systemic immunosuppressive therapy for eye diseases study. Ophthalmology. 2013;120:186–92. https://doi.org/10.1016/j.ophtha.2012.07.052.
Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom-Guillaume S, Edelsten C, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018;77:1107–17. https://doi.org/10.1136/annrheumdis-2018-213131.
Leal I, Steeples LR, Wong SW, Giuffre C, Pockar S, Sharma V, et al. Update on the systemic management of non-infectious uveitis in children and adolescents. Surv Ophthalmol. 2023. https://doi.org/10.1016/j.survophthal.2023.01.002.
Heiligenhaus A, Minden K, Tappeiner C, Baus H, Bertram B, Deuter C, et al. Update of the evidence based, interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Semin Arthritis Rheum. 2019;49:43–55. https://doi.org/10.1016/j.semarthrit.2018.11.004.
Cunningham ET Jr. Exogenous factors influencing endogenous inflammation: what can patients do to improve control of their own uveitis? Br J Ophthalmol. 2010;94:813–4. https://doi.org/10.1136/bjo.2009.178780.
Al-Haddad C, BouGhannam A, Abdul Fattah M, Tamim H, El Moussawi Z, Hamam RN. Patterns of uveitis in children according to age: comparison of visual outcomes and complications in a tertiary center. BMC Ophthalmol. 2019;19:137. https://doi.org/10.1186/s12886-019-1139-5.
de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003;87:879–84. https://doi.org/10.1136/bjo.87.7.879.
Markomichelakis NN, Aissopou EK, Chatzistefanou KI. Pediatric non-infectious uveitis: long-term outcomes and complications. Ocul Immunol Inflamm. 2023;24:1–8. https://doi.org/10.1080/09273948.2022.2162422.
Cann M, Ramanan AV, Crawford A, Dick AD, Clarke SLN, Rashed F, et al. Outcomes of non-infectious paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol. 2018;16:51. https://doi.org/10.1186/s12969-018-0266-5.
Mohammed MA, Moles RJ, Chen TF. Medication-related burden and patients’ lived experience with medicine: a systematic review and metasynthesis of qualitative studies. BMJ Open. 2016;6:e0100356. https://doi.org/10.1136/bmjopen-2015-010035.
Sav A, Kendall E, McMillan SS, Kelly F, Whitty JA, King MA, et al. ‘You say treatment, I say hard work’: treatment burden among people with chronic illness and their carers in Australia. Health Soc Care Community. 2013;21:665–74. https://doi.org/10.1111/hsc.12052.
Ashkenazy N, Saboo US, Robertson ZM, Cao J. The effect of patient compliance on remission rates in pediatric noninfectious uveitis. J AAPOS. 2019;23:334.e1–6. https://doi.org/10.1016/j.jaapos.2019.08.280.
Freedman RB, Jones SK, Lin A, Robin AL, Muir KW. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch Ophthalmol. 2012;130:306–11. https://doi.org/10.1001/archopthalmol.2011.1788.
World Health Organization. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. https://apps.who.int/iris/handle/10665/42682.
Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child. 2010;95:717–23. https://doi.org/10.1136/adc.2009.175125.
Bender B, Wamboldt FS, O’Connor SL, Rand C, Szefler S, Milgrom H, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85:416–21. https://doi.org/10.1016/s1081-1206(10)62557-4.
Lievens CW, Gunvant P, Newman J, Gerstner M, Simpson C. Effect of proview self-tonometry on pharmaceutical compliance. Clin Exp Optom. 2006;89:381–5. https://doi.org/10.1111/j.1444-0938.2006.00081.x.
El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharm. 2016;82:268–79. https://doi.org/10.1111/bcp.12942.
Wang J, Racette L, Ott P, Donaldson D, Neeley DE, Pager DA. Objectively-measured compliance to atropine penalization treatment in children with amblyopia: a pilot study. J Eye Sci. 2016;31:146–52. https://doi.org/10.3978/j.issn.1000-4432.2016.09.13.
Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26:155–9. https://doi.org/10.5001/omj.2011.38.
Landier W, Chen Y, Hageman L, Kim H, Bostrom BC, Casillas JN, et al. Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: a Children’s Oncology Group study. Blood. 2017;129:1919–26. https://doi.org/10.1182/blood-2016-07-726893.
Al-Hassany L, Kloosterboer SM, Dierckx B, Koch BCP. Assessing methods of measuring medication adherence in chronically ill children–a narrative review. Patient Prefer Adherence. 2019;13:1175–89. https://doi.org/10.2147/PPA.S200058.
Chieng R, Coutsouvelis J, Poole S, Dooley MJ, Booth D, Wei A. Improving the transition of highly complex patients into the community: impact of a pharmacist in an allogeneic stem cell transplant (SCT) outpatient clinic. Support Care Cancer. 2013;21:3491–5. https://doi.org/10.1007/s00520-013-1938-9.
Moore DB, Neustein RF, Jones SK, Robin AL, Muir KW. Pediatric glaucoma medical therapy: who more accurately reports medication adherence, the caregiver or the child? Clin Ophthalmol. 2015;9:2209–12. https://doi.org/10.2147/OPTH.S93038.
Aston J, Patel N, Smuels J, Aujla T, Malesi G, Huynh C. Patient/Carers’ recollection of medicines related information from an out-patient clinic appointment. Abstracts from the Neonatal and Paediatric Pharmacists Group (NPPG), 21st Annual Conference. Arch Dis Child. 2016;101:e2. https://doi.org/10.1136/archdischild-2016-311535.53.
Stanbury RM, Graham EM. Systemic corticosteroid therapy: side effects and their management. Br J Ophthalmol. 1998;82:704–8. https://doi.org/10.1136/bjo.82.6.704.
Becker I, Horneff G. Risk of serious infection in juvenile idiopathic arthritis patients associated with tumor necrosis factor inhibitors and disease activity in the German biologics in pediatric rheumatology registry. Arthritis Care Res. 2017;69:552–60. https://doi.org/10.1002/acr.22961.
Davis AM, Graham TB, Zhu Y, McPheeters ML. Depression and medication nonadherence in childhood-onset systemic lupus erythematosus. Lupus. 2018;27:1532–41. https://doi.org/10.1177/09612033187797103.
Gatwood JD, Johnson J, Jerkins B. Comparisons of self-reported glaucoma medication adherence with a new wireless device: a pilot study. J Glaucoma. 2017;26:1056–61. https://doi.org/10.1097/IJG.0000000000000777.
Carbone L, Zebrack B, Plegue M, Joshi S, Shellhaas R. Treatment adherence among adolescents with epilepsy: what really matters. Epilepsy Behav. 2013;27:59–63. https://doi.org/10.1016/j.yebeh.2012.11.047.
Ariceta G, Lara E, Camacho JA, Oppenheimer F, Vara J, Santos F, et al. Cysteamine (Cystagon®) adherence in patients with cystinosis in Spain: successful in children and a challenge in adolescents and adults. Nephrol Dial Transpl. 2015;30:475–80.
Walders N, Drotar D, Kercsmar C. The allocation of family responsibility for asthma management tasks in African-American adolescents. J Asthma. 2000;37:89–99. https://doi.org/10.3109/02770900009055432.
Acknowledgements
The study was sponsored by Manchester Foundation Trust.
Funding
The study was funded through a grant awarded by the British and Irish Paediatric Ophthalmology and Strabismus Association (BIPOSA).
Author information
Authors and Affiliations
Contributions
EKYG—conception, study design, data collection, writing original draft and subsequent revisions. OM—data collection, writing review and editing. LS—data collection, writing review and editing. JLA—conception, supervision, study design, data collection, writing review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Green, E.K.Y., McGrath, O., Steeples, L. et al. Monitoring compliance to topical therapy in children and young people with uveitis. Eye 38, 572–577 (2024). https://doi.org/10.1038/s41433-023-02736-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02736-0