Abstract
Objective
To assess sex-specific risk factors for Graves’ orbitopathy (GO) in newly diagnosed Graves’ disease (GD) patients.
Methods
A retrospective cohort study was conducted using the National Health Insurance Service’s sample database, which consisted of 1,137,861 subjects from 2002 to 2019. The international classification of disease-10 codes was used to identify those who developed GD (E05) and GO (H062). A multivariable Cox proportional hazards model was used to estimate the effect of risk factors on GO development.
Results
Among 2145 male and 5047 female GD patients, GO occurred in 134 men (6.2%) and 293 women (5.8%). A multivariable Cox regression model revealed that GO development was significantly associated with younger age (HR = 0.84, 95% CI = 0.73–0.98), low income (HR = 0.55, 95% CI = 0.35–0.86), and heavy drinking (HR = 1.79, 95% CI = 1.10–2.90) in men, and with younger age (HR = 0.89, 95% CI = 0.81–0.98), lower body mass index (HR = 0.55, 95% CI = 0.33–0.90), high total cholesterol (HR = 1.04, 95% CI = 1.01–1.06), hyperlipidaemia (HR = 1.37, 95% CI = 1.02–1.85), and lower statin dose (HR = 0.37, 95% CI = 0.22–0.62) in women. There was no association between smoking and GO development in both men and women.
Conclusions
The risk factors for GO development were sex-dependent. These results show the need for more sophisticated attention and support considering sex characteristics in GO surveillance.
Similar content being viewed by others
Introduction
Graves orbitopathy (GO) is an autoimmune disorder mainly associated with Graves’ disease (GD) [1]. GO involves inflammatory changes in the orbital tissues and presents as variable signs and symptoms, including foreign body sensation, lid swelling, and exophthalmos. It is disfiguring, can produce visual disturbances, and seriously deteriorate the quality of life of patients. Although a more detailed understanding of the pathogenesis of GO has led to the investigation of various therapeutic drugs, the long-term adverse effects of GO have not yet been prevented. The identification and assessment of risk factors for the development of GO in GD patients will be of great help in alleviating patients’ anxiety and determining rational treatment strategies.
Previous studies have reported that insufficient control of hyperthyroidism [2], high thyroid-stimulating hormones receptor antibody levels (TSH-R-Ab) [3], smoking [4, 5], radioactive iodine (RAI) treatment [6, 7], and high cholesterol levels [8, 9] are established risk factors for the development of GO in patients with GD. However, few studies have been conducted to analyse these risk factors by sex. As is widely known, the prevalence of GO demonstrates a noteworthy, sex-specific characteristics—women are three to four times more likely to develop the disease than men [10]. However, men develop GO at an older age and suffer from worse clinical features despite a lower incidence [11]. Typically, sex differences in the incidence and clinical features suggest that the risk factors for GO may also differ between men and women. However, the aforementioned risks of GO development are mainly reported from studies on Western populations. Studies on the risk of GO occurrence in Asians have generally been conducted at the level of individual hospitals, and relatively few studies have been conducted at multiple centres or nationwide.
The purpose of this study was to identity sex-specific risk factors for GO development in patients with new-onset GD in Koreans. In the process of exploring risk factors for GO, we attempted to overcome hospital-level data limitation by conducting a nationwide, population-based, large-scale, cohort study.
Materials and methods
Data source
This is a retrospective cohort study using the National Health Insurance Service (NHIS)’s sample database, which consisted of 1,137,861 subjects from 2002 to 2019. The NHIS is the public medical insurance system managed by the Korean government, providing a compulsory insurance system for all residents in Korea. The NHIS also regularly provides free, standardized health checkups for all insured persons. This study was approved by the Institutional Review Board of Chung-Ang University Hospital (IRB No. 2107-035-19376), and informed consent was waived. This study adheres to the guidelines of the Declaration of Helsinki.
Study population
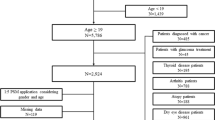
New-onset GD patients were defined as subjects who were diagnosed at least twice with international classification of disease (ICD)-10 codes (E05) and who were prescribed a medication for hyperthyroidism for more than 180 days. Such new-onset GD patients were identified between 2004 and 2018. Those diagnosed with GD for the first time after 2019 or those diagnosed with GO before 3 months prior to being diagnosed with GD were excluded. Patients diagnosed with thyroid cancer with ICD-10 code (C73) were also excluded. A total of 7192 new-onset GD patients were eligible for our study (Fig. 1).
Definition of outcomes and variables
GO patients were defined as those diagnosed with ICD-10 code (H062). Patents with active GO were defined as those who were prescribed intravenous steroids for more than 6 times within three months from the initial prescription of intravenous steroids since the GO diagnosis.
The health examination provided by NHIS includes survey responses and screening measurement values. Information on income level, residence, alcohol consumption, and smoking history was obtained at baseline through standardized, self-administered questionnaires collected within 3 years before the GD diagnosis. By income decile, those in the top ~30% were classified as high-income, those in the bottom ~30% as low-income, and the rest as middle-income. Residence was categorized into three categories: capital, metropolitan, and rural. Alcohol consumption was categorized into three categories: non-drinker, mild-to-moderate drinker (1–5 times a week and less than 7 drinks on any day per week), and heavy drinker (more than 6 times a week or more than or equal to 7 drinks on any day per week) [12]. Smoking status was classified as nonsmoker and ex- or current smoker. The body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). After an overnight fast, blood was drawn to measure blood glucose (mg/dL) and total cholesterol (mg/dL). The degree of control of GD could not be determined because thyroid function and TSH-R-Ab tests are not included in the health examination items of the NHIS.
We identified cases with comorbidities, including diabetes mellitus (DM), hyperlipidaemia, and autoimmune disease. DM was defined as subjects who were diagnosed at least twice with ICD-10 codes (E11–E14) or who had been prescribed oral hypoglycaemic agents or insulin. Hyperlipidaemia was defined as subjects diagnosed at least twice with ICD-10 codes (E78) or who had been prescribed lipid-lowering medication. Autoimmune disease was defined as subjects diagnosed with ICD-10 codes (rheumatoid arthritis M06, polymyalgia rheumatica M353, multiple sclerosis G35, type 1 diabetes mellitus E10, systemic lupus erythematous M32, sarcoidosis D86, Sjogren syndrome M350, myasthenia gravis G700, pernicious anaemia D51, autoimmune haemolytic anaemia D591, autoimmune adrenalitis D271). RAI treatment was defined as having a sodium iodide drug code. Thyroidectomy was defined as one of the following surgical codes: P4551, P4552, P4553, P4554, and P4561. In addition, among lipid-lowering drugs, statins were separately analysed. Exposure to statins was defined as having at least two prescriptions of atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, or simvastatin and was assessed at baseline and during the follow-up periods. The dose of each statin with an equivalent LDL cholesterol reduction effect for the various statins was calculated as 80 mg fluvastatin, 40 mg lovastatin, 2 mg pitavastatin, 40 mg pravastatin, 5 mg rosuvastatin, and 20 mg simvastatin based on 10 mg of atorvastatin [13, 14].
To address the aspect of time-varying comorbidities, history of RAI, and statin dose over time, time-dependent covariates for those variables were created for each month during the follow-up periods. We calculated the average daily statin dose per month by obtaining the average value of the daily atorvastatin equivalent statin dose (mg) each month. The codes used to identify disease and medications are available in the Supplementary Tables (Table S1 and Table S2).
Statistical analysis
Descriptive statistics are shown as median (interquartile ranges) or mean ± standard deviation for continuous variables and percentage for categorical variables. The chi-square test or Fisher’s exact test was used for comparisons of categorical variables. Student’s t test or Mann–Whitney U test was used for comparisons of continuous variables at baseline and the end of follow-up. To summarize the change in statin dose for ever-statin users over the follow-up periods, the average daily statin doses per year were described and a linear regression was fitted for the statin doses to characterize a linear trend in the statin doses.
There were missing data in the health examination data. Multiple imputation using chained equations (MICE) were used to generate 10 imputed datasets with predictive mean matching for continuous data and logistic regression for binary data [15]. A multivariable Cox proportional hazards model was used to estimate the effects of risk factors on GO development, including baseline demographic, behaviour variables, and time-dependent variables of comorbidities, history of RAI, and statin doses with a time scale of a month. Subgroup analyses were performed according to sex to estimate the sex-specific hazard ratios (HRs) using multiply imputed data for each sex. To evaluate the effect modification by sex, we included interactions between sex and risk factors in the Cox models and performed a significance test for those interaction effects. In each analysis, the estimates were pooled using Rubin’s rule [16]. All GD patients included in the study were followed from the date of first diagnosis of GD to either the date of GO diagnosis, death, lost-to-follow-up, or December 31, 2019. For the subgroup analysis, active GO was assessed from the date of GO diagnosis to either the date of death, lost-to-follow-up, or December 31, 2019, for GO patients. We used the SAS software ver. 9.4 (SAS Institute, Cary, NC, USA) and R ver. 3.3 3 (R Foundation for Statistical Computing). For all analyses, p < 0.05 was considered statistically significant.
Results
A total of 7192 GD patients were included in the cohort—2.4 times more women (70.2%) than men (29.8%). Among 2145 male and 5047 female GD patients, GO occurred in 134 men (6.2%) and 293 women (5.8%), indicating no sex-related difference in incidence. The median period of follow-up of the study participants was 94 (50–140) months for men and 102 (55–148) months for women.
Table 1 presents the characteristics of the study population according to sex. The median age of GD diagnosis was 40 (31–51) years for men and 41 (28–50) years for women among the GD patients with GO, which was younger than that of the GD patients without GO (p = 0.049, p < 0.001 respectively). The median time from the first GD diagnosis to the first GO diagnosis was 9 months for both men and women. At the time of diagnosis of GD, men had a higher incidence of GO in the low-income category and in the case of heavy drinkers, but there was no difference in women according to income or alcohol consumption. In women, higher BMI, comorbidity of DM, and taking statins were associated with a reduced risk of GO development (p = 0.024, p = 0.021, p = 0.014 respectively). Smoking was not associated with the risk of GO development in both men and women.
To evaluate how the statins are related to GO development, changes in the statin dose over time were compared in both men and women. In both men and women, the statin dose continued to increase in GD patients without GO and also tended to increase in female GD patients with GO. In contrast, the statin dose tended to decrease over time in men with GO. Moreover, both men and women patients with GO showed a severe fluctuation in statin dose compared to patients without GO (Fig. 2).
In a multivariable Cox regression model, younger age (HR = 0.84, 95% CI = 0.73–0.98, p = 0.022), low income (HR = 0.55, 95% CI = 0.35–0.86, p = 0.009), heavy drinking (HR = 1.79, 95% CI = 1.10–2.90, p = 0.019) were significantly associated with GO development in men, while younger age (HR = 0.89, 95% CI = 0.81–0.98, p = 0.018), lower BMI (HR = 0.55, 95% CI = 0.33–0.90, p = 0.019), high total cholesterol (HR = 1.04, 95% CI = 1.01–1.06, p = 0.003), and hyperlipidaemia (HR = 1.37, 95% CI = 1.02–1.85, p = 0.039) were associated with GO development in women. In women who were satin users, a higher statin dose was significantly associated with a reduced risk of GO development (HR = 0.37, 95% CI = 0.22–0.62, p < 0.001) (Table 2). The HRs of individual risk factors calculated by the multivariable regression analysis were displayed as a Forest plot according to the sex of the patients; only the statin dose showed a significant difference in HRs between men and women (Fig. 3 and Table S3).
There were 60 (14.1%) patients with active GO out of 427 patients with GO. There was no significant difference in the occurrence of active GO according to sex (p = 0.616). In patients who progressed to active GO, the median ages at the time of GD diagnosis were 49 (44–59) years for men and 47 (38–53) years for women, which were significantly older than those of inactive GO patients (p = 0.005, p = 0.009 respectively). In men, the proportion of statin users at the time of GD diagnosis was significantly higher in active GO patients than in inactive GO patients (Table S4).
Discussion
GO is known to have a higher prevalence in women than in men. In this study, GD was 2.4 times more common in women than in men, but the incidence of GO in GD patients was similar in both sexes—6.2% in men, and 5.8% in women. The higher incidence of GO in women may be attributed to the higher prevalence of GD in women and the shared pathogenesis of both diseases. Although there have been studies on the effects of sex hormones on thyroid function and sex-related differences in iodine intake in thyroid disease [17, 18], such sex-related differences have not been studied as risk factors for developing GO. Therefore, we tried to identify sex-specific risk factors for GO through a large population-based cohort study. As a result, our regression model showed that younger age, low income, and heavy drinking were associated with GO development in men. In women, younger age, high serum total cholesterol, and lower BMI at the time of GD diagnosis, and hyperlipidaemia after GD diagnosis were associated with GO development. In addition, higher statin doses in women statin users after GD diagnosis were significantly associated with a reduced risk of GO development. Interestingly, in women, factors related to metabolic disorder, such as hyperlipidaemia, hypercholesterolemia, and statin use, were identified as the primary risk factors, while in men, sociobehavioural factors were associated with GO development.
Previous studies have reported that high levels of total and LDL-cholesterol correlated with the presence of GO and the clinical activity score [8, 9]. Our results also indicated that the comorbidity of hyperlipidaemia and serum total cholesterol were significantly associated with GO development in women. Both were found to be insignificant or even to reduce GO risk in a simple comparison, but the combination in a cox regression model revealed that there was a significant association with GO development in women. These conflicting results between the simple comparison and the regression model may be due to longer follow-up in patients without GO than in patients with GO whose follow-up was discontinued after the occurrence of GO according to our study protocol. Usually, the incidence of hyperlipidaemia gradually increases with age, which may be the case in patients without GO who had a longer follow-up period in our study. The confounding effect of the simultaneous use of statins to treat hyperlipidaemia may also be another reason. Statins were negatively correlated with GO development in our study. Hyperlipidaemia and statin use in men did not show any significant association with GO risk in the regression model, despite their association in a simple comparison. Total cholesterol can also have a positive correlation with age. In this study, the characteristics of women patients with GO with younger age and lower BMI may have influenced the reverse result in simple comparison compared to the regression model with a significant relationship between total cholesterol levels and GO development. The mechanism by which hypercholesterolemia or hyperlipidaemia induces GO development is not clear, but it may be associated with systemic, chronic inflammation caused by impaired lipid metabolism.
As hypercholesterolemia emerges as a risk factor for GO, there is growing interests in determining whether GO development can be reduced by statin use. Statins have anti-inflammatory effects and are expected to inhibit the cascade of inflammatory cell and cytokine activation in GO [19, 20]. Moreover, the inhibition of adipogenesis by statins may suppress GO symptoms. In this study, after confirming the preventive effect of statins on GO development, the equivalent statin dose was calculated to determine the differences in dose-response according to sex. Higher doses of statins were associated with a higher GO risk in men (p = 0.05) but a significantly lower GO risk in women (p < 0.001). Considering that statin dose tended to increase over time in GD patients without GO and in women GO patients (Fig. 2), women GD patients with good adherence to statin use seemed to have a much lower risk of developing GO than statin non-users, suggesting the protective effect of statin use. However, it is difficult to explain the relationship between statin dose and the risk of GO in men, although compliance may be one of the reasons, considering that statin dose decreases with time in male GO patients. Therefore, the apparent sex-related differences in statin doses observed in the Forest plots may reflect differences in physiological response or may be due to differences in drug compliance between the sexes.
In men, low income and heavy drinking were found to be associated with the occurrence of GO. A previous study revealed that low social grade and higher social deprivation were associated with more severe GO [21]. People with low socioeconomic status may have fewer opportunities to seek appropriate treatment in hospitals or may be more reluctant to receive treatments. Our study did not perform the thyroid function test for these subjects, but it is likely that GD may not be well controlled in these patients, causing an acceleration in GO occurrence. As is widely known, uncontrolled GD is an important risk factor for GO development. Diet may also be important. Oxidative stress is known to result in the production of free radicals, leading to the proliferation and inflammation of orbital fibroblasts in GO [22]. Patients with lower incomes may have lower intakes of antioxidants in the food they consume [21]. Heavy drinking is also estimated to worsen this lack of antioxidants [23]. It is difficult to explain why these socioeconomic factors are particularly related to the occurrence of GO in men rather than women. Our findings highlight the importance of improving access to high-quality care for these patients.
Smoking is the risk factor most consistently associated with the onset and severity of GO [24]. However, our study did not find a significant association between smoking and GO development in both men and women. In men, 76.4% of GO patients were past or current smokers with high smoking rates, but it did not reach the statistical significance (p = 0.077). Presumably, high proportion (68.3%) of smokers in GD patients contributed to the failure of deriving statistical significance. In contrast, in women, only 6.2% of the GD patients were smokers, so it is estimated that the number was insufficient to analyse the effect of smoking on GO development. The social environment in which smoking is more widespread in men than women seems to have influenced the results. Alternatively, these results could be because only the smoking status, but not the amount or period of smoking, was analysed.
RAI treatment is a well-known risk factor for GO deterioration [25] and is therefore, administered more cautiously to GD patients with prophylactic oral corticosteroids. Our study did not show any significant association between RAI treatment and GO development. The proportion of patients with GD who received RAI treatment in this study was relatively low, less than 7%. However, Korean doctors give priority to antithyroid drug treatment for the treatment of GD, with ~8% of patients receiving RAI treatment [26]. It is possible that the low RAI treatment rate contributed to the relatively low incidence of GO. Changes in clinical practice to the concomitant use of steroids during RAI treatment in patients with high risk of GO are also presumed to be responsible for the relatively low GO occurrence. Thyroidectomy was also not associated with GO development in GD patients. Several studies have reported that thyroidectomy is neutral to the natural course of GO [27, 28].
Abnormal levels of serum thyroid hormone are associated with GO occurrence, and proper management of thyroid dysfunction is paramount. Insufficient regulation or rapid changes in thyroid function affect GO progression. Serum TSH-R-Ab helps in assessing and predicting the response of antithyroid treatment, but it has also been reported as an important risk factor related to the severity and activity of GO [29]. However, this study was conducted based on NHIS’s claims data, and detailed serum measurements could not be analysed because NHIS does not collect any data on thyroid function tests or TSH-R-Ab titer. Thus, the lack of data on the control of GD, which is an important risk factor for GO, is a limitation of this study. Further research is needed to determine whether GD control is affected by sex.
Only older age in both sexes and statin use in men were associated with the progression to active GO in this study, but it was difficult to perform regression analysis due to the small numbers of relevant risk factors and patients with active GO. Previous studies have reported that older age, male sex, and smoking can affect the severity of GO [11, 30]. Although smoking did not show any statistical significance, our results demonstrated that the older age was related to the progression of active GO in both men and women.
This study has several limitations. First, this was a retrospective cohort study, where our data could only suggest the risk factors associated with GO development: the causative relationship could not be predicted. Second, since our study used insurance data consisting of diagnostic codes, there might be a possibility of misdiagnosis. In our study, GO occurred in ~6% of GD patients, which was lower than in previous studies [31]. Patients with mild GO may have been excluded as GO was defined using only the diagnostic code, and it is possible that the incidence of GO was underestimated. Finally, as our subjects consisted mostly of Koreans, our results might not be applicable to other ethnicities. However, the present study was a population-based, nationwide, large-scale cohort study with many advantages to offset its limitations. Moreover, time-dependent analyses for time-varying variables such as statin use and comorbidities were performed to minimize guarantee-time bias.
In conclusion, this is the first study to consider sex-related characteristics as risk factors for GO development. We found that younger age, low income, and heavy drinking were associated with GO development in men, while younger age, lower BMI, high total cholesterol, hyperlipidaemia, and lower statin doses in statin users among women were associated with GO development. Further studies are necessary to determine the causal relationship between these risks and GO development and to evaluate whether management of these risks modifies GO progression.
Supplementary information is available at Eye’s website.
Summary
What was known before
-
Graves’ orbitopathy (GO) is three to four times more likely to develop in women than in men. Uncontrolled hyperthyroidism, high TSH receptor antibody, smoking, radioactive iodine treatment, and high cholesterol levels have been reported as risk factors for the development of GO in patients with Graves’ disease (GD).
What this study adds
-
GO development was associated with younger age, low income, and heavy drinking in men, and with younger age, low BMI, high total cholesterol, hyperlipidaemia, and lower doses of statin in statin users in women. GO patients had more fluctuation in statin dose than GD patients without GO in both men and women, and women GD patients with good adherence to statin use had a lower risk of developing GO than statin non-users.
Data availability
The data for this study is available from the corresponding author on reasonable request.
References
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L. Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest. 2013;36:444–9.
Prummel MF, Wiersinga WM, Mourits MP, Koornneef L, Berghout A, van der Gaag R. Effect of abnormal thyroid function on the severity of Graves’ ophthalmopathy. Arch Intern Med. 1990;150:1098–101.
Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–70.
Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013;79:145–51.
Khong JJ, Finch S, De Silva C, Rylander S, Craig JE, Selva D, et al. Risk factors for graves’ orbitopathy; the Australian thyroid-associated orbitopathy research (ATOR) study. J Clin Endocrinol Metab. 2016;101:2711–20.
Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell’Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–8.
Träisk F, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700–7.
Lanzolla G, Sabini E, Profilo MA, Mazzi B, Sframeli A, Rocchi R, et al. Relationship between serum cholesterol and Graves’ orbitopathy (GO): a confirmatory study. J Endocrinol Invest. 2018;41:1417–23.
Sabini E, Mazzi B, Profilo MA, Mautone T, Casini G, Rocchi R, et al. High serum cholesterol is a novel risk factor for graves’ orbitopathy: results of a cross-sectional study. Thyroid. 2018;28:386–94.
Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch Ophthalmol. 1993;111:197–201.
Perros P, Crombie AL, Matthews JN, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf). 1993;38:367–72.
Cho YK, Kang YM, Yoo JH, Lee J, Park JY, Lee WJ, et al. Implications of the dynamic nature of metabolic health status and obesity on risk of incident cardiovascular events and mortality: a nationwide population-based cohort study. Metabolism. 2019;97:50–6.
Kendrach MG, Kelly-Freeman M. Approximate equivalent rosuvastatin doses for temporary statin interchange programs. Ann Pharmacother. 2004;38:1286–92.
Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35:139–51.
van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94.
Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–98.
Al-Jabri A, Cooke J, Cournane S, Healy ML. Gender differences in estimating I-131 thyroid uptake from Tc-99m thyroid uptake for benign thyroid disease. Br J Radiol. 2021;94:20200700.
Tahboub R, Arafah BM. Sex steroids and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:769–80.
Stein JD, Childers D, Gupta S, Talwar N, Nan B, Lee BJ, et al. Risk factors for developing thyroid-associated ophthalmopathy among individuals with Graves disease. JAMA Ophthalmol. 2015;133:290–6.
Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87.
Edmunds MR, Huntbach JA, Durrani OM. Are ethnicity, social grade, and social deprivation associated with severity of thyroid-associated ophthalmopathy. Ophthalmic Plast Reconstr Surg. 2014;30:241–5.
Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Lee SM, et al. Increased response to oxidative stress challenge in Graves’ ophthalmopathy orbital fibroblasts. Mol Vis. 2011;17:2782–8.
Hillbom M. Oxidants, antioxidants, alcohol and stroke. Front Biosci. 1999;4:e67–71.
Tellez M, Cooper J, Edmonds C. Graves’ ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf). 1992;36:291–4.
Stan MN, Durski JM, Brito JP, Bhagra S, Thapa P, Bahn RS. Cohort study on radioactive iodine-induced hypothyroidism: implications for Graves’ ophthalmopathy and optimal timing for thyroid hormone assessment. Thyroid. 2013;23:620–5.
Chung JH. Antithyroid drug treatment in Graves’ disease. Endocrinology and metabolism (Seoul, Korea). 2021;36:491–9.
Bartalena L. The dilemma of how to manage Graves’ hyperthyroidism in patients with associated orbitopathy. J Clin Endocrinol Metab. 2011;96:592–9.
Liu ZW, Masterson L, Fish B, Jani P, Chatterjee K Thyroid surgery for Graves’ disease and Graves’ ophthalmopathy. Cochrane Database Syst Rev. 2015:Cd010576.
Diana T, Kahaly GJ. Thyroid stimulating hormone receptor antibodies in thyroid eye disease-methodology and clinical applications. Ophthalmic Plast Reconstr Surg. 2018;34:S13–s9.
Li Q, Ye H, Ding Y, Chen G, Liu Z, Xu J, et al. Clinical characteristics of moderate-to-severe thyroid associated ophthalmopathy in 354 Chinese cases. PLoS One. 2017;12:e0176064.
Chin YH, Ng CH, Lee MH, Koh JWH, Kiew J, Yang SP, et al. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin Endocrinol (Oxf). 2020;93:363–74.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1A2C1011351) and the Chung-Ang University Research Grants in 2021. The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
LJ was responsible for planning, extracting and analysing data, and writing the paper. KJ was responsible for extracting and analysing data. AHY was responsible for conceptualization, interpretation of results, and review of the paper. LJK was responsible for planning, conceptualization, interpretation of results, and review of the paper. All listed authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J., Kang, J., Ahn, H.Y. et al. Sex-specific risk factors associated with graves’ orbitopathy in Korean patients with newly diagnosed graves’ disease. Eye 37, 3382–3391 (2023). https://doi.org/10.1038/s41433-023-02513-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02513-z
This article is cited by
-
An Appraisal of the Preventive Effect of Statins on the Development of Graves’ Ophthalmopathy: A Hospital-Based Cohort Study
Ophthalmology and Therapy (2024)