Abstract
Objectives
To evaluate the macular vascular flow in eyes with idiopathic macular pucker (EyeiMP), pre and post pars plana vitrectomy with epiretinal and limiting membranes peeling, and to compare it with the vascular flow in the healthy fellow eyes (Eyefellow), taken as physiological reference value.
Methods
40 eyes of 40 patients were recruited. Best-corrected visual acuity (BCVA) was evaluated. Spectral domain optical coherence tomography (SD-OCT) and OCT-angiography parameters were central foveal thickness (CFT), choroidal thickness (CT), foveal avascular zone (FAZ) area, vessel area density (VAD), vessel length fraction (VLF), vessel density index (VDI) of superficial capillary plexus (SCP) and deep vascular complex (DVC), choriocapillaris (CC) flow. Absolute and relative difference calculation was applied to evaluate macular vascular flow in EyeiMP adjusted for physiological changes detected in Eyefellow. Follow-up: 6 months.
Results
BCVA improved (p = 0.003) in all cases following surgery. CFT reduced postoperatively (p = 0.0138). FAZ area was smaller in EyeiMP than Eyefellow (p = 0.0071) preoperatively and postoperatively it shrank further (p = 0.0027). After surgery, inverse correlation between FAZ area and BCVA was detected (r-0.683). VAD of SCP was pre- and post-operatively higher in EyeiMP than Eyefellow (baseline p = 0.0344, 6th month p = 0.0466). Relative difference of VDI of SCP (p = 0.0096) and CC flow (p = 0.0013) at 6 months reduced. DVC flow changed significantly only in Eyefellow. CT increased post-operatively in both EyeiMP (p = 0.0345) and Eyefellow (p = 0.00423), but relative difference did not change.
Conclusions
Vascular flow indices monitoring demonstrated significant changes in both eyes: EyeiMP and Eyefellow. Relative difference of vascular flow provided objective estimate of changes due to iMP surgery taking into account physiological changes in Eyefellow.
Similar content being viewed by others
Introduction
Surgical peeling of epiretinal membrane (ERM) and internal limiting membrane (ILM) is the standard treatment for idiopathic macular pucker (iMP) [1, 2]. Several studies have demonstrated the effectiveness of surgery in functional recovery and morphological restoration of the foveal profile; however, less is known about the effects on retinal and choroidal circulation [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Optical coherence tomography angiography (OCTA) has been used to evaluate the retinal and choroidal vascular flow changes in eyes affected by iMP before and after surgery showing conflicting results [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Furthermore, the ocular vascular flow may be affected by the patient’s general conditions. A realistic measurement of vascular flow changes due to iMP should consider the physiological fluctuations occurring regardless of iMP. To date, there are no normative data for age, sex and race about flows and density of capillaries in any of the instruments currently in use. Therefore, we propose to use the healthy fellow eye as a reference value of ‘relative flow’. The relative flow calculation, by correction with fellow healthy eye parameters, eliminates the interindividual variability of a possible age-matched group. Furthermore, choosing the fellow eye as control would ensure that both eyes would still be influenced by the effect of the same systemic conditions if any present. Monitoring healthy fellow eye vascular flow offers an estimate of physiological fluctuations and allows to have a more objective estimation of the vascular flow changes related to iMP. The vascular flow changes corrected for physiological values can be estimated in two ways: the absolute and the relative difference. Absolute difference gives you the real number difference of vascular flow between the eye with iMP and the healthy fellow eye: vascular flow index of eye with iMP—vascular flow index of healthy fellow eye. Relative difference calculates the changes of one value relatively to another reference value. Specifically, it calculates the percentage change of ratio between the macular vascular flow values of eye with iMP and of healthy fellow eye, in order to determine how close they are, relative to the healthy fellow eye vascular flow taken as physiological reference value. It gives a direct insight into the true scale of difference between the eye with iMP and healthy fellow eye: vascular flow of eye with iMP—vascular flow of fellow eye/vascular flow of fellow eye × 100. This approach allows to differentiate situations of equal absolute difference but with different physiological reference value (healthy fellow eye). In other words, a preoperative greater vascular flow value in eye with iMP or a postoperative increase of vascular flow in eye with iMP must be analysed in the context of the physiological reference value. If the physiological vascular flow is low, the increase of vascular flow in iMP will be greater than in the condition in which the physiological vascular flow is high. On the other hand, if the physiological value was a constant, not subject to interindividual variability, it would allow the same quantitative change, therefore the relative difference would not be more informative than the absolute one. An example of calculation of absolute and relative difference is shown in Fig. 1a.
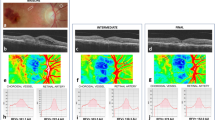
a Graph shows a calculation of the absolute and relative difference. Healthy fellow eye (Eyefellow) has a flow of 7 units at baseline, 8 units at the first control, and 9 units at the last control. Eye with idiopathic macular pucker (EyeiMP) has a flow of 4 units at baseline, 5 units at the first control, and 6 units at the last control. The absolute difference (EyeiMP-Eyefellow) is constant, i.e., it is 3 units for each follow-up timepoints. The relative difference varies from 75% at baseline, 60% at the first control and 50% at the last control. While the absolute difference is an algebraic subtraction between absolute values, the relative difference is a percentage of change in EyeiMP to reference values given by the Eyefellow. b Spectral domain optical coherence tomography (SD-OCT). Left: Automatic segmentation of the internal limiting membrane (ILM) and the retinal pigment epithelium/Bruch’s membrane (RPE/BM) for the measurement of central foveal thickness (CFT), represented on the ETDRS grid. Right: manual segmentation of the outer choroidoscleral boundary for the measurement of choroidal thickness (CT), represented on the ETDRS grid. c En-face OCTA. Foveal avascular zone (FAZ) area of superficial capillary plexus (SCP), automatically calculated by the software Angioscan 8 OCT angiography (OCTA) on a macular 3 × 3 mm map. d B-scan OCTA. Top: Superficial capillary plexus (SCP), generated as a layer extending from the internal limiting membrane (ILM) to a segmentation line located 12 μm above the inner plexiform layer/inner nuclear layer (IPL/INL) interface on a macular 3 × 3 mm map. Bottom: Deep vascular plexus (DVC), generated as a layer extending from 8 μm above the IPL/INL interface to a deeper line 12 μm above the outer plexiform layer/outer nuclear layer (OPL/ONL) on a macular 3 × 3 mm map, including the intermediate (ICP) and the deep capillary plexuses (DCP). e En-face OCTA image of the choriocapillary (CC) layer. Isolation of the CC layer by the identification of a slab of 8 μm from 33 μm posteriorly to the segmentation of the retinal pigment epithelium/Bruch’s membrane (RPE/BM) on a macular 3 × 3 mm map. f OCTA image after elaboration with FIJI software (imageJ version 1.52e extension, National Institutes of Health, Bethesda, Maryland, USA): automatic local thresholding analysis performed with the Phansalkar method using a radius of 15 pixels available in the aforementioned version of the software, according to Spaide’s method [21, 22]. Automatic distribution of the obtained data into 15 categories in a range from 1 to 100 pixels, distributed on a logarithm graph number of areas vs. logarithm amplitude of areas of flow voids (FV). The resulting scatter chart can be intersected by a trend line described by the mathematical relationship described by Spaide: log (number of areas) = m log (amplitude of areas) + b. The slope of regression line is represented by the absolute angular coefficient “m” value, that increases for larger choriocapillary (CC) streams.
The Authors’ purpose of this study is to evaluate the macular vascular flow variations in eyes affected by iMP pre and post pars plana vitrectomy (PPV) and peeling of ERM and ILM, comparing and correcting them with those of the healthy fellow eyes.
Materials and methods
All patients affected by iMP in one eye (EyeiMP) and healthy fellow eye (Eyefellow), who underwent PPV combined to ERM and ILM peeling, between March 2018 and March 2020, at the department of Ophthalmology of University of Padova, Italy, were consecutively recruited. All research and measurements adhered to the tenets of the Declaration of Helsinki, the project has been approved by our review board (SEV/376) and written informed consent was obtained for each participant. The diagnosis of iMP was based on the slit lamp biomicroscope according to grade 2 of Gass classification [17] considering iMP the ERM, detectable as a distinct greyish membrane on the inner surface associated or not to retinal folds, traction lines, vascular tortuosity or dilation. The diagnosis was confirmed by spectral domain optical coherence tomography (SD-OCT) RS 3000 Advance (Nidek Gamagori, Japan) according to the criteria proposed by Govetto et al. [18]. An important inclusion criterion was the pseudophakic status of EyeiMP, to avoid that the status of the natural lens may have had any effect on the level of visual acuity. To fulfill this criterion, the eye should have undergone cataract surgery at least 6 months before the recruitment for the study, so to reduce any possible influence of the surgery on the ocular vascular flow. Exclusion criteria were poor eye fixation, nystagmus, central serous chorioretinopathy, drusen, glaucoma or an intraocular pressure (IOP) higher than 24 mmHg, axial length (AL) longer than 26 millimeters (mm) and cases of ERM secondary to other diseases (i.e., retinal and choroidal inflammation, vascular occlusion, ocular trauma, diabetes mellitus, retinal detachment, severe myopia, uveitis). The indication for surgery was based on clinical presentation, best-corrected visual acuity (BCVA) impairment and referred symptoms, like metamorphopsia, diplopia, micropsia, macropsia and photophobia. Demographic parameters were the following: age (years), gender (male, female), eye (right, left), AL (mm). BCVA, reported in Snellen chart and converted in logarithm of minimum angle of resolution (logMAR) for the statistical analysis, and IOP were recorded at baseline, 1-, 3-, and 6 months post-op. Surgery was performed by a single surgeon (RF) using a 27-gauge system (Constellation PC Vision Enhancement System, Alcon).
All our patients followed a standard preoperative protocol between a few days or weeks preceding surgery including blood tests (full blood count, electrolytes, glucose, HbA1c, creatinine, coagulation screening (PT/PTT), fibrinogen, thyroid function, blood group), electrocardiogram, and chest X-ray if over 65 years of age or if smoking. An anaesthetic review ensured an overall view of the patients’ state of health and indication of suitability for surgery. A supra-aortic trunk color-doppler echo was not routinely performed, unless risk factors for cerebrovascular diseases were known or found, according to the Italian guidelines. Since this diagnostic test is considered the first choice for the diagnosis of cerebrovascular diseases, the presence of pathological alterations in the flow of the supra-aortic trunks entailed the unsuitability for macular pucker surgery and the surgery postponement up to the resolution of the vascular pathology.
Physiological fluctuations must be taken into consideration and relative flow calculation by correction with fellow eye parameters is a fundamental advancement when a method (OCT-angiography) aims to study vascular modification of eye before and after surgery. Unfortunately, the study of flows (density of capillaries) has no normative reference values for age or sex or race in any of the instruments currently in use. Therefore, we proposed the “relative flow” by using the flow parameters of the healthy fellow eye as reference values. The relative flow calculation (by correction with fellow healthy eye parameters) eliminates the need to compare results with a normal age-matched group. This comparison approach results more reliable by reducing interindividual variability, and it is the method/approach described in many other published studies due to the lack of normative data flow.
Spectral domain optical coherence tomographic (SD-OCT) and OCT angiography (OCTA) study
SD-OCT and OCTA parameters were measured preoperatively and during follow-up (at baseline, 1, 3, 6 months post-op) in EyeiMP and Eyefellow. SD-OCT scans were collected by two operators (LT and IG) using SD-OCT RS 3000 Advance (Nidek Gamagori, Japan). The images were acquired in the morning, between 10.00 and 12.00 o’clock. According to the protocol, a linear macular scan, six radial scans and a macular map of 3 × 3 mm centered onto the fovea were collected.
SD-OCT parameters
Two SD-OCT parameters were measured: central foveal thickness (CFT) and choroidal thickness (CT). CFT was automatically measured as represented on the ETDRS grid in the central subfield macular thickness (Fig. 1b left). CT was measured manually on each of the 6 radial scans, between the line representing the retinal pigment epithelium (RPE, the outermost hyper-reflective retinal layer) and the line representing the outer choroidoscleral boundary (the outer hyper-reflective line of the choroid). Average CT of subfoveal, nasal, temporal, superior, and inferior sectors was calculated (Fig. 1b right). Postoperative incidence of cystoid macular edema (CME) was evaluated.
OCTA parameters
The foveal avascular zone (FAZ) area of superficial capillary plexus (SCP) was automatically calculated by the Nidek OCTA software (Fig. 1c).
Study of retinal capillary plexi
The software Angioscan 8 OCTA was used for the acquisition of OCTA image. The enface image of SCP is generated as a layer extending from ILM to a segmentation line located 12 micrometers (μm) above the inner plexiform layer/inner nuclear layer (IPL/INL) interface (Fig. 1d). Deep vascular complex (DVC) extends from 8 μm above the IPL/INL interface to a deeper line 12 μm above the outer plexiform layer/outer nuclear layer (OPL/ONL), including intermediate capillary plexus (ICP) and deep capillary plexus (DCP) (Fig. 1d). Poor-quality images were excluded according to the automated image quality indicators generated by the OCTA software, scan quality index (SQI) inferior to 2/5 and signal strength index (SSI) inferior to 8/10. To quantify each bi-dimensional en-face image, three quantitative parameters were analysed with FIJI software (imageJ version 1.52e extension, National Institutes of Health, Bethesda, Maryland, USA): vessel area density (VAD), vessel length fraction (VLF) and vessel density index (VDI) [19, 20]. With imageJ, the enface OCTA images were converted into a binarized image: VAD (the total image area occupied by the vessels) was calculated by dividing the number of black pixels counted by the software in the binarized image by the total number of image pixels (868 × 868 = 753,424). From the binarized image was then automatically iteratively deleted the outer boundary until a single pixel for each vessel segment remained, obtaining a skeletonized image. VLF (which represents the length of all the vessels) was calculated dividing the number of black pixels in the skeletonized image by the total number of image pixels. Both the binarized and the skeletonized images were processed to calculate the average vessel caliber, obtaining the VDI parameter [19, 20].
Study of choriocapillaris (CC) flow
From the same OCTA scan, through manual segmentation, choriocapillary (CC) layer was isolated, by the identification of a slab of 8 μm from 33 μm posteriorly to the segmentation of the retinal pigment epithelium (RPE)—Bruch’s membrane complex (Fig. 1e). With FIJI software an automatic local thresholding analysis was performed with the Phansalkar method, using a radius of 15 pixels available in the aforementioned version of the software, according to Spaide’s method [21, 22]. The obtained data were automatically distributed into 15 categories in a range from 1 to 100 pixels and, once imported into an Excel spreadsheet (Microsoft Office; Microsoft Inc, Redmond, Washington, USA), distributed on a logarithm graph number of areas vs. logarithm amplitude of areas. The resulting scatter chart can be intersected by a trend line described by the mathematical relationship described by Spaide [21, 22]: log (number of areas) = m log (amplitude of areas) + b. This relationship provided us an objective proxy of blood flow value, the absolute angular coefficient value “m” that could be compared between different images of the same subject. This value increases for larger CC flow streams (Fig. 1f).
Analysis data and statistical methods
Sample size
The principal hypothesis of the study was the evaluation of vascular flow changes after 6 months of surgery. Paired t test was used to statistically evaluate the changes of absolute difference between iMP and fellow eyes measures as well as their relative difference defined as the ratio of absolute difference over the measure on fellow eye. For a two-sided test with 95% confidence level (type I error alpha = 0.0.5) and a power 1-beta = 0.80, a sample size of at least 34 patients was necessary to evaluate a medium effect size of ES = 0.50. Sample size was determined by the software G*power 3.1.
Statistics
Study parameters were summarized according to usual methods of descriptive statistics: absolute and relative (percentage) frequencies for qualitative variables (gender, eye) and mean, standard deviation, range for quantitative variables (age, BCVA, AL, IOP, vascular flow indices). Changes over time of vascular flow indices in EyeiMP and Eyefellow were analysed. The absolute and relative difference of vascular flow indices were calculated. Normal distribution of parameters was verified by Shapiro–Wilk’s test. Changes over time of quantitative variables were tested by means of paired t test when normal distribution was assumed, otherwise Wilcoxon signed-rank test was used. All analyses were performed using SAS® 9.4 (SAS Institute, Cary, NC, USA) and test results were interpreted as statistically significant if p < 0.05.
Results
Participants
Forty eyes of 40 consecutive patients affected by iMP who underwent surgery were collected. Table 1a shows the demographic and preoperative data of patients. After surgery, in EyeiMP BCVA improved significantly from baseline to each time point of follow-up (p = 0.003). IOP did not change significantly in the postoperative period (Table 1b).
SD-OCT parameters
CFT reduced significantly in EyeiMP from baseline (432.8 ± 80 μm) to each timepoint of follow-up up to 6 months postop months (359.1 ± 48.3 μm) (p = 0.0138) (Fig. 2a). No statistical difference of CT was preoperatively detected between EyeiMP and Eyefellow. After surgery, significant increase of CT was detected in both EyeiMP and Eyefellow, respectively from 198.39 ± 57.8 μm at baseline to 213.45 ± 48.3 μm at 6 months postop (p = 0.0345) in EyeiMP and from 214.72 ± 47.5 μm at baseline to 233.02 ± 52.3 μm at 6 months postop in Eyefellow (p = 0.00423). Absolute and relative difference of CT did not change significantly during the follow-up (Fig. 2b). No cases of postoperative CME were detected.
a Variation of central foveal thickness (CFT) in eyes with idiopathic macular pucker (EyeiMP) and in healthy fellow eyes (Eyefellow) from baseline to each time points of the follow-up. b Variation of choroidal thickness (CT) in eyes with idiopathic macular pucker (EyeiMP) and in healthy fellow eyes (Eyefellow) during the follow-up, absolute and relative difference. c Variation of foveal avascular zone area (FAZ area) in eyes with idiopathic macular pucker (EyeiMP) and in healthy fellow eyes (Eyefellow) during the follow-up. d Variation of choriocapillaris (CC) flow in eyes with idiopathic macular pucker (EyeiMP) and in healthy fellow eyes (Eyefellow) during the follow-up, absolute and relative difference.
OCTA parameters
Preoperatively FAZ area was significantly smaller in EyeiMP than Eyefellow, 0.11 ± 0.1 mm2 in EyeiMP and 0.24 ± 0.13 mm2 in Eyefellow (p = 0.0071). After surgery, FAZ area of EyeiMP shrank further, with significant reduction at 6 months postop (p = 0.0027) (Fig. 2c). A postoperative inverse correlation between FAZ area and BCVA was detected (r-0.683). The comparison of vascular flow indices of SCP between EyeiMP and Eyefellow showed that VAD of SCP was preoperatively higher in EyeiMP (p = 0.0344). After surgery, VAD in EyeiMP was significantly higher at 1 (p = 0.0123) and 6 months (p = 0.0466), VLF and VDI were significantly higher at 1 month postop, respectively p = 0.0196 and p = 0.0171, compared to Eyefellow. A significant increase of VDI from baseline to 1 month postop in EyeiMP (p = 0.0045), and from baseline to 3 (p = 0.0061) and 6 months postop (p = 0.018) in Eyefellow were detected. No significant changes of absolute difference of VAD, VLF and VDI from baseline to each timepoints of follow-up were detected. Relative difference of VDI decreased significantly at 6 months, (p = 0.0096) (Fig. 3). No statistical differences of pre- and postoperative vascular flow indices of DVC were detected between EyeiMP and Eyefellow. The vascular flow indices of DVC in EyeiMP did not change significantly from baseline to each timepoint of follow-up. On the contrary significant changes of VAD and VLF of DVC in Eyefellow were detected, respectively from baseline to 3 (p = 0.0205) and 6 months postop (p = 0.001) for VAD and from baseline to 1 (p = 0.0116) and 3 months postop (p = 0.03) for VLF (Fig. 4). Absolute difference showed a significant postoperative change at 6 months for VAD (p = 0.001). No relative difference changes were detected during the follow-up. CC flow did not differ preoperatively between EyeiMP and Eyefellow. In the postop follow-up a significant lower CC flow was detected in Eyefellow compared to EyeiMP at 3 months (p = 0.0481). CC flow of EyeiMP significantly reduced from baseline to each timepoints of follow-up, from −0.68 ± 0.11 at baseline to −0.52 ± 0.13 at 6 months postop (p = 0.0013). The absolute difference did not change significantly during the follow-up. A significant change of relative difference from baseline to 6 months postop was highlighted (p = 0.04) (Fig. 2d).
Discussion
In our cases series, eyes with iMP were associated with a higher CFT and a contracted FAZ area compared to healthy fellow eyes. All patients presented a visual impairment associated with metamorphopsia. PPV with ERM and ILM peeling was effective, BCVA improved in all patients and CFT reduced significantly. These data confirm the results reported by many authors. However, after surgery a further reduction of FAZ area was detected. Although there is a common agreement about a reduction of FAZ area in EyeiMP due to the centripetal contraction caused by iMP [3,4,5,6,7,8,9], the previous published results about the effects of surgery on FAZ area are conflicting. Some authors registered its enlargement after ERM and ILM peeling [3, 4, 8,9,10]. Of these, some assumed that FAZ area enlargement is a positive recovery sign, by interpreting it as a natural response to a release of mechanical centripetal traction of ERM; others, assumed that FAZ area enlargement is expression of a damage to Müller cells and subsequently to perifoveal capillary plexi [8,9,10]. Opposite results were reported by Kumagai et al. who noticed a postoperative contraction of FAZ area with a centripetal displacement of the capillaries towards the optic nerve head, suggesting that ILM could stretch the retina centrifugally or that peeling could displace the retina by causing structural changes on Müller cells [9]. Kim et al. reported a reduction of FAZ area associated with worse postoperative BCVA [8]. In the current study, FAZ area of EyeiMP was smaller than Eyefellow and it further shrank postoperatively. Although BCVA improved significantly at the end of follow-up, an inverse correlation between FAZ area and BCVA was demonstrated: in other words, the more the FAZ area shrank, the worse was the BCVA. We hypothesize that ILM peeling may induce a retinal displacement and consequent contraction of FAZ. This could be the cause of metamorphopsia and diplopia, sometimes postoperatively referred by patients and often underestimated in face of an improvement of BCVA and a tomographically re-establishment of the normal foveal profile. Regarding the retinal vascular plexi, the traction induced by ERM may cause a distortion of retinal vessels causing an imbalanced distribution of vessel density [8, 10,11,12,13,14,15,16, 20,21,22,23,24,25]. In the current study, the vascular flow indices of SCP show a not-unique trend, on the contrary no changes of vascular flow indices of DVC were demonstrated before and after surgery, leading to hypothesize that DVC was not involved by iMP. In SCP, VAD was higher in EyeiMP than Eyefellow before and after surgery. VAD is expression of the percentage of area occupied by vessels on OCTA images. Thus, a higher preoperative VAD in EyeiMP may have been induced by centripetal tractions of iMP with vessels displacement toward the macula, and the detected high VAD postoperatively, may be the result of centripetal displacement of the retina induced by peeling. This finding is supported by the detection of shrank FAZ area previously described. No preoperative statistical difference of VDI and VLF of SCP between EyeiMP and Eyefellow was demonstrated, leading to the deduction that iMP did not affect these indices. VLF did not change after surgery, on the contrary VDI changed in both EyeiMP and the Eyefellow. Although the absolute difference of VDI did not change significantly, the relative difference at 6 months was significantly reduced compared to the baseline, which means that VDI in EyeiMP increased postoperatively reaching values similar to Eyefellow, i.e., it normalized. Only the calculation of the relative difference made it possible to highlight the significant change of VDI, which would not have emerged from the evaluation of the absolute values of VDI. Although no significant changes of vascular flow indices of DVC were demonstrated before and after surgery, it can be noted that preoperatively the VAD is lower and the VDI higher in EyeiMP than in Eyefellow. The Authors interpret these data by hypothesizing that a lower VAD is the consequence of the tractions induced by the ERM inducing a retinal stress, specifically on the Muller cells; the higher VDI can be an expression of a vascular dilation secondary to an inflammatory process induced by the suffering of the retinal tissue. Observing the postoperative trend of VAD and VDI of EyeIMP, there is an improvement trend of these vascular indices which tend to get close to those of Eyefellow. This trend could mean that surgical treatment leads to an improvement in the vascular flow of DVC (Fig. 4).
CC flow in EyeiMP varied significantly from baseline to postoperative time, while Eyefellow flow did not show significant changes. At baseline CC flow in EyeiMP was higher than in Eyefellow, in the postop follow-up it decreased, confirmed by the analysis of the changes of relative difference (Fig. 2d). This means that CC flow in EyeiMP tends to reach values like those of the Eyefellow, i.e., it tends to normalize. An increase of CC flow may be secondary to inflammatory process induced by iMP. CC flow is one of the highest of any vascular bed of the human body and that it largely lacks autoregulation [3, 17]. CC flow is, per unit mass, three to four times higher than that in the kidney [19]. The most important function of the CC is to sustain adequate bidirectional mass exchange to deliver the metabolites sustaining the photoreceptors and to remove waste from the outer retina. Thus, the increased flow could be the consequence of a greater load of disposal of catabolic substances and of a greater intake of chemical mediators of inflammation. Removal of ERM results in a reduction of this inflammatory process with a normalization of the CC flow. The analysis of postoperative changes of CT in both EyeiMP and Eyefellow showed a significant increase up to 6 months. On the contrary, the analysis of relative difference changes did not show significant results, in contrast with the previous studies that reported a decrease of CT postoperatively [12, 13, 15, 16]. However, the results analysed in previously published paper were based exclusively on the comparison of absolute values of CT between EyeiMP and Eyefellow or control eyes, i.e., they did not consider the physiological changes of vascular flow in each patient. In our study changes of CT were detected also during the monitoring of Eyefellow. Furthermore, the analysis of relative difference of CT demonstrated that there were no significant changes during the chosen follow-up.
Monitoring the FAZ area, CFT and VAD of SCP provided valuable information about the effective postoperative release of tractions and the retinal detente following ERM removal. The restoration of VDI of SCP and CC flow in EyeiMP at values like Eyefellow prove the beneficial effects of the iMP surgery. The SCP is the plexus directly involved by the iMP and a postoperative restoration of the vessels’ diameter at values similar to Eyefellow is an expression of an improvement in vascular flow. Regarding the CC, the flow reduction can be interpreted as a reduction in the inflammatory process due to iMP. The evaluation of the SD-OCT and OCTA parameters in both eyes of patients affected by macular pucker in one eye and with the healthy fellow eye allowed to demonstrate that the changes of macular vascular flow in EyeiMP are not sufficient to estimate how much surgery can affect the retinal and choroidal vascular flow. The relative difference showed a reduction in the VDI at 6 postoperative months which would not have emerged from the analysis of the absolute values which showed significant variations in the Eyefellow but not in the EyeiMP. The significant postoperative increase in CT in EyeiMP, highlighted by the analysis of the absolute values, is denied by the relative difference calculation which considered the significant changes of CT that also occurred in the Eyefellow. Finally, where the vascular flow did not change in Eyefellow, i.e., in CC, the relative difference confirmed a significant change of CC flow in EyeiMP detected also by the analysis of the absolute values. The relative difference allows to have an objective estimate of the macular vascular flow due to a disorder or to a surgical treatment, corrected for the physiological changes of vascular flow occurring independently of the disorder itself, in both eyes.
Study limitations
The limitations of the study are related to the challenging proposal itself and therefore to the measurement of the physiological retinal vascular flow and to its variations secondary to the pathology. The study calculation had to consider various factors and use a rigorous statistical method to evaluate the vascular flow. Another limitation of the study is the lack of the supra-aortic trunks color-doppler to confirm normal blood flow to both eyes from the inner carotid artery. Our study was not delivered in the settings of a clinical trial, therefore only patients with specific cardiovascular risks were undergoing a color-doppler following anaesthetic review. Nevertheless, all our patients followed a systemic standard preoperative protocol to be able to identify possible patients at risk.
The limited number of patients recruited is due to the strict inclusion criteria such as the healthy contralateral eye and relatively recent pseudophakia in the eye with iMP. A larger study is needed to confirm the authors’ hypotheses regarding the vascular flow changes detected. However, the aim of the study is to propose a new methodology to estimate the macular vascular flow that is accurate and precise.
Conclusions
Providing the relative difference calculation within the OCTA software would make its use more immediate and available to ophthalmologists less exposed to this specific field. The integration would improve its performance and would be immediately available with no need of complex calculations once the scans of both eyes have been acquired by the machine. The new software may allow automatically quantification of the vascular flow and when applied on a large scale, it may allow normalized data by age and sex. Furthermore, this methodology could be easily analysed and adopted as a new automated tool by an artificial intelligence algorithm possibly unveiling entirely new biomarkers useful in the diagnosis and treatment of retinal diseases.
Summary
What was known before
-
OCTA has been used to evaluate the retinal and choroidal vascular flow changes in eyes affected by iMP before and after surgery showing conflicting results.
What this study adds
-
The vascular flow indices monitoring demonstrated significant changes in both eyes: EyeiMP and the fellow Eye (Eyefellow).
-
Relative difference of vascular flow provided objective estimate of changes due to iMP surgery considering physiological changes in Eyefellow.
Data availability
Data are available upon request and are with the first author.
References
Pournaras CJ, Emarah A, Petropoulos IK. Idiopathic macular epiretinal membrane surgery and ILM peeling: Anatomical and functional outcomes. Semin Ophthalmol. 2011;26:42–6. https://doi.org/10.3109/08820538.2010.544237.
Rahman R, Stephenson J. Early surgery for epiretinal membrane preserves more vision for patients. Eye. 2014;28:410–4. https://doi.org/10.1038/eye.2013.305.
Yoon YS, Woo JM, Woo JE, Min JK. Superficial foveal avascular zone area changes before and after idiopathic epiretinal membrane surgery. Int J Ophthalmol. 2018;11:1711–5. https://doi.org/10.18240/ijo.2018.10.21.
Kitagawa Y, Shimada H, Shinojima A, Nakasashizuka H. Foveal avascular zone area analysis using optical coherence tomography angiography before and after idiopathic epiretinal membrane surgery. Retina. 2019;39:339–46. https://doi.org/10.1097/IAE.0000000000001972.
Kumagai K, Furukawa M, Suetsugu T, Ogino N. Foveal avascular zone area after internal limiting membrane peeling for epiretinal membrane and macular hole compared with that of fellow eyes and healthy controls. Retina. 2018;38:1786–94. https://doi.org/10.1097/IAE.0000000000001778.
Okawa Y, Maruko I, Kawai M, Hasegawa T, Arakawa H, Lida T. Foveal structure and vasculature in eyes with idiopathic epiretinal membrane. PLoS ONE. 2019;14:1–8. https://doi.org/10.1371/journal.pone.0214881.
Romano MR, Cennamo G, Schiemer S, Rossi C, Sparnelli F, Cennamo G. Deep and superficial OCT angiography changes after macular peeling: idiopathic vs diabetic epiretinal membranes. Graefes Arch Clin Ophthalmol. 2017;255:681–9. https://doi.org/10.1007/s00417-016-3534-4.
Kim YJ, Kim S, Lee JY, Kim JG, Yoon YH. Macular capillary plexuses after epiretinal membrane surgery: an optical coherence tomography angiography study. Br J Ophthalmol. 2017;102:1086–91. https://doi.org/10.1136/bjophthalmol-2017-311188.
Kumagai K, Ogino N, Furukawa M, Ooya R, Horie E. Early centripetal displacements of capillaries in macular region caused by internal limiting membrane peeling. Clin Ophthalmol. 2018;12:755–63. https://doi.org/10.2147/OPTH.S158826.
Chen H, Chi W, Cai X, Deng Y, Jiang X, Wei Y, et al. Macular microvasculature features before and after vitrectomy in idiopathic macular epiretinal membrane: an OCT angiography analysis. Eye. 2019;33:619–28. https://doi.org/10.1038/s41433-018-0272-3.
Mastropasqua L, Borrelli E, Carpineto P, Toto L, Di Antoio L, Mattei PA, et al. Microvascular changes after vitrectomy with internal limiting membrane peeling: an optical coherence tomography angiography study. Int Ophthalmol. 2018;38:1465–72. https://doi.org/10.1007/s10792-017-0608-1.
Casini G, Lazzeri S. Analysis of choroidal thickness change after 25-gauge vitrectomy for idiopathic epiretinal membrane with or without phacoemulsification and intraocular lens implantation. Ophthalmologica. 2017;237:78–84. https://doi.org/10.1159/000452769.
Ahn SJ, Woo SJ, Park KH. Choroidal thickness change following vitrectomy in idiopathic epiretinal membrane and macular hole. Graefes Arch Clin Exp Ophthalmol. 2016;254:1059–67. https://doi.org/10.1007/s00417-015-3154-4.
Yu Y, Teng Y, Gao M, Liu X, Chen J, Liu W. Quantitative choriocapillaris perfusion before and after vitrectomy in idiopathic epiretinal membrane by optical coherence tomography angiography. Ophthalmic Surg, Lasers Imaging Retin. 2017;48:906–15. https://doi.org/10.3928/23258160-20171030-06.
Michalewska Z, Michalewski J, Ornafel-Sagan K, Navrocki J. Swept-source optical coherence tomography correlations between retina and choroid before and after vitrectomy for epiretinal membranes. Am J Ophthalmol. 2016;165:100–7. https://doi.org/10.1016/j.ajo.2016.02.003.
Michalewska Z, Michalewski J, Adelman R, Zawislak E, Navrocki J. Choroidal thickness measured with swept source optical coherence tomography before and after vitrectomy with internal limiting membrane peeling for idiopathic epiretinal membranes. Retina. 2015;35:487–91.
Gass JDM. Macular dysfunction caused by epiretinal membrane contraction. In: Stereoscopic atlas of macular diseases: diagnosis and treatment. 4th ed., vol. 2. St Louis, Mo: Mosbyy; 1997. p. 938–50.
Govetto A, Lalane RA III, Sarraf D, Figueroa MS, Hubschman JP. Insights into epiretiinal membranes: presence of ectopic inner foveal layers and a neww optical coherence tomography staging scheme. Am J Ophthalmol. 2017;175:99–113. https://doi.org/10.1016/j.ajo.2016.12.006.
Reif R, Qin J, An L, Zhi Z, Dziennis S, Wang R. Quantifying optical microangiography images obtained from a spectral domain optical coherence tomography system. Int J Biomed Imaging. 2012;2012:509783. https://doi.org/10.1155/2012/509783.
Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57:OCT362.70. https://doi.org/10.1167/iovs.15-18904.
Phansalkar N, More S, Sabale A, Joshi M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. 2011 International Conference on Communications and Signal Processing, 2011, p. 218–220. https://doi.org/10.1109/ICCSP.2011.5739305.
Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58–67. https://doi.org/10.1016/j.ajo.2016.07.023.
Zouache MA, Eames I, Klettner CA, Luthert PJ. Form, shape and function: segmented blood flow in the choriocapillaris. Sci Rep. 2016;6:35754. https://doi.org/10.1038/srep35754.
Aleksic M, Matoussevitch V, Heckenkamp J, Brunkwall J. Changes in internal carotid blood flow after CEA evaluated by transit-time flowmeter. Eur J Endoscopic Surg. 2006;31:14–7. https://doi.org/10.1016/j.ejvs.05.08.029.
Eckstein HH, Eichbaum M, KlemmK, Doerfler A, Ringleb P, Bruckner T, et al. Improvement of carotid blood flow after carotid endarterectomy—evaluation using intraoperative ultrasound flow measurement. Eur J Vasc Endovasc Surg. 2003;25:168–74. https://doi.org/10.1053/ejvs.2002.1820.
Author information
Authors and Affiliations
Contributions
RF—conceptualization, writing of the paper, supervision. LT, IG, and JYS—collection and analysis of the data, writing of the paper. BP—formal analysis, review and editing. GDS—writing of the paper, review. AM—data curation, supervision. All authors have read and agreed to the published version of the paper.
Corresponding author
Ethics declarations
Competing interests
RF, LT, IG, JYS, BP, and AM certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials. GDS received honoraria and consultancies fees outside the current work from the following companies: Allergan Abbvie, Apellis, Bayer, Boehringer Ingelheim, Heidelberg Engineering, Novartis.
Ethics approval and consent to participate
All research and measurements adhered to the tenets of the Declaration of Helsinki and written informed consent was obtained for each participant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frisina, R., De Salvo, G., Tozzi, L. et al. Effects of physiological fluctuations on the estimation of vascular flow in eyes with idiopathic macular pucker. Eye 37, 1470–1478 (2023). https://doi.org/10.1038/s41433-022-02158-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02158-4