Abstract
Purpose
Standard corneal collagen cross-linking (S-CXL) is an effective treatment to arrest Keratoconus (KC) progression in children. Less is known on the long-term efficacy of accelerated CXL (A-CXL) in paediatric populations.
Methods
A historical cohort analysis of paediatric patients (≤18 years) with KC who underwent S-CXL and A-CXL at two tertiary referral centres in Israel between 2010–2017. Preoperative and 3-year postoperative evaluation included changes in visual acuity (best spectacle corrected [BSCVA]) and uncorrected [UCVA]), refractive errors, and keratometric data.
Results
Ninety-three eyes of 93 patients were analysed (A-CXL: n = 39; S-CXL: n = 54). Baseline characteristics were similar between groups. Both groups showed a significant improvement in visual acuity compared to baseline (S-CXL: 0.810–0.602 LogMAR UCVA; A-CXL: 0.890–0.306 LogMAR UCVA, p < 0.05 for both). Improvement in BSCVA and UCVA following A-CXL was non-inferior to S-CXL (< ± 0.2 LogMAR). Kmax decreased by a mean of 0.98 ± 5.56 dioptres following S-CXL (p = 0.02) and by 1.48 ± 8.4 dioptres following A-CXL (p = 0.015). Thinnest pachymetry decreased following both treatments (S-CXL: by 26.8 ± 40.7 µm, p = 0.001, A-CXL: by 10.2 ± 13.4 µm, p = 0.028), the difference between groups was within the non-inferiority margin (< ± 10 µm).
Conclusions
Paediatric patients followed for three years after A-CXL showed improved visual function, reduced corneal astigmatism and Kmax, and decreased thinnest corneal thickness. A-CXL was non-inferior to S-CXL at three years in terms of best-corrected and uncorrected visual acuity, thinnest pachymetry, and astigmatism. For Kmax, non-inferiority could not be concluded.
Similar content being viewed by others
Introduction
Keratoconus (KC) is the most common primarycorneal ectasia, characterized by corneal steepening, apical thinning, and scarring. KC results in a progressive visual impairment which can be unamenable to spectacles, require the use of rigid contact lenses, corneal implants, and may eventually require keratoplasty [1]. KC prevalence in the paediatrics population was noted to be between 1:200 and 1:25 [2, 3].
Corneal collagen cross-linking (CXL) with riboflavin and ultraviolet (UV)-A light has emerged as a successful treatment to halt KC progression in children. CXL treatment induces additional covalent bonds between collagen fibrils and proteoglycan core proteins which stiffens the cornea and increases its rigidity and stability [4]. This results in halting KC progression and, over time, to continued improvement in keratometry and visual parameters [5]. Soeters et al. compared the paediatric population after CXL to the adolescence and adult populations and showed more corneal flattening and more corrected distance visual acuity improvement in children [6].
The standard CXL protocol (S-CXL, Dresden Protocol), first reported in 2003, includes 30 min of UV-A radiation. An accelerated protocol (A-CXL) has been introduced in clinical practice to shorten treatment time, improve compliance and reduce general anaesthetic use [7]. It is based on the Bunsen–Roscoe law of reciprocity, which allows treatment time to be shortened by increasing the radiation intensity to deliver the same total energy dose [8]. Hence, compared to S-CXL, A-CXL uses greater UVA irradiance intensity with lower exposure time (10 min of 9 mW/cm2 = 30 min of 3 mW/cm2 as both protocol results at the end total cumulative energy dose of 5.4 J/cm2) [9]. Limited data is available assessing the outcomes of A-CXL in children [9,10,11,12]. Only four studies directly compared the two and were generally limited by short follow-up, Supplementary Table 1 [9,10,11]. Therefore, in this study, we aimed to review the outcomes following A-CXL and S-CXL with an extended follow-up (3 years) and assess whether A-CXL is equivalent to S-CXL in terms of visual acuity refraction, tomography parameters, and rate progression, regression, and stability.
Methods
Study design and population
We retrospectively reviewed KC paediatric patients’ files who underwent S-CXL and A-CXL at Shamir Medical Center and Soroka University Medical Center in Israel between 2010–2017. Patients were included if they were 18 years of age or younger, at the time of corneal cross-linking (CXL), and had at least 36 months of follow-up after the procedure. We excluded patients with a history of any ocular disease or surgery, Kmax over 69 dioptres (D), central corneal thickness less than 400 microns (μm), history of recurrent corneal erosion or dystrophies, history of corneal herpes virus infection, history of rheumatological and autoimmune disease, or sensitivity to any of the substance that used in CXL procedure. Children with Kmax >69 D and pachymetry <400 were excluded as in many cases these corneas cannot undergo regular CXL and need a different approach.
KC diagnosis and progression were made using Pentacam (Pentacam, Oculus, Wetzlar, Germany). Progression was defined as a 1.5D increase in mean keratometric value or 1D increase in Kmax or decreased 5% in central corneal thickness at two consecutive evaluations by Pentacam [13]. Patients with progressive KC were treated with either S-CXL (before 2015) or A-CXL (after 2015) protocol.
The study was conducted following the tenets of the Declaration of Helsinki, and approval was obtained from the local institutional review board in both centres. Parents gave their written informed consent in a general consent form, provided by the Israel Ophthalmology Society and the Israel Cornea Working Group. While this form included only general information about the risk and benefit of the CXL procedure, we discussed in detail the specific A-CXL protocol and the data that was available at the time”.
Surgical technique
CXL was performed under topical anaesthesia, with oxybuprocaine hydrochloride 0.4% eye drops [13] used before the procedure. An 8.0-mm diameter of the central corneal epithelium was removed using a blunt spatula or epithelial peeler. Then, iso-osmolar riboflavin (Medio-Cross 0.1%; Peschke Meditrade GmbH, Huenenberg, Switzerland) was instilled every 5 min for 30 min. UV-A was then irradiated at an intensity of 3 mW/cm2 for 30 min (S-CXL) or 9 mW/cm2 for 10 min (A-CXL). Riboflavin solution was instilled continuously every 2 min during UV-A irradiation. The patient was instructed to fixate on the light source, and the surgeon constantly monitored adequate centration. All eyes were bandaged immediately after the procedure. Table 1 Summarized the CXL methods.
Primary and secondary outcomes
Preoperative 1-year and 3-year postoperative evaluation included changes in visual acuity (best and uncorrected in LogMAR units), refractive errors (spherical equivalent, spherical error, and cylindrical error), and keratometric data (Ksteep front, Kflat front, Kmean front, Kmax, corneal astigmatism front and back, thickness at the thinnest point). Postoperative progression was defined as an increase of 1.00 D or more in Kmax, regression was defined as a reduction of 1.00 D or more, and stabilization was defined as no change of more than 1.00 D [10].
Statistical analysis
We used SPSS version 25 (IBM, Chicago, USA) and MedCalc Statistical Software version 14.8.1 (Ostend, Belgium) for all statistical analyses. The Shapiro–Wilk test was used to evaluate normal distributions. Normally distributed continuous variables are presented as means ± standard deviations and were compared using a T-test. Non-normally distributed continuous variables are presented as median (interquartile ranges) and were compared using the Mann–Whitney U test. Categorical variables are presented as percentages and were compared using chi-squared or Fisher’s test as appropriate. All statistical analyses performed were two-sided and statistical significance was set at a p value of 0.05. For analysis of non-inferiority, the difference in treatment outcomes between accelerated and standard CXL was calculated at 1 and 3 years along with 95% confidence intervals (95% CI). Confidence intervals that did not cross the non-inferiority margin (or did in favour of accelerated CXL) indicated non-inferiority of accelerated CXL in these outcomes. Non-inferiority margins for thinnest pachymetry were set to ±10 µm, for Kmax and corneal astigmatism (front) to ±1 dioptre and visual acuity to ±0.2 LogMAR (equivalent to two ETDRS lines) [14].
Results
Demographics
Ninety-three eyes of 93 patients were included in the study, 54 eyes in the S-CXL group and 39 eyes in the A-CXL group. The mean age was 15.60 ± 2.29 years (range 8–18). Baseline characteristics were similar between groups except for BSCVA, which was better in the S-CXL group (0.27 ± 0.17 LogMAR vs. 0.44 ± 0.23 LogMAR, p = 0.02). Table 2 shows the demographic and clinical characteristics of both groups prior to the CXL. No complications were reported in either group during the three-year follow-up period, including infection, delayed re-epithelialization, or corneal scarring.
Visual acuity
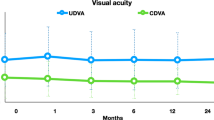
The average BSCVA and UCVA at baseline, 12 and 36 months after treatment of both groups is demonstrated in Fig. 1. Both groups showed a significant improvement in visual acuity three years after the CXL compared to baseline (p < 0.05).
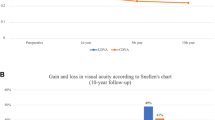
Mean BSCVA slightly decreased following S-CXL at 1-year by −0.223 ± 0.26 LogMAR and following A-CXL improved by 0.1307 ± 0.242 LogMAR. The difference between treatment types at 1-year was −0.153 LogMAR (95% CI: −0.257 to −0.48), showing non-inferiority of A-CXL as seen in Fig. 2. The difference was −0.152 LogMAR (95% CI: −0.254 to −0.048), demonstrating A-CXL’s non-inferiority after three years from treatment.
The tall solid line is the mean difference between accelerated and standard CXL, and the two flanking solid lines indicate the 95% confidence interval for the difference. The dashed line indicates the non-inferiority margin, and the solid thin line marks zero (no difference). Notice that for thinnest pachymetry, UCVA and BSCVA at 1 year and 3 years, and for astigmatism, at 3-years, the confidence interval does not cross the non-inferiority margin (or crosses in favour of accelerated CXL), showing non-inferiority of accelerated CXL in these outcomes.
Mean UCVA at 1-year slightly improved following both treatment types, after S-CXL by 0.158 ± 0.42 LogMAR and after A-CXL by 0.160 ± 0.45. The mean difference between the two was relatively small at −0.0017 LogMAR (95% CI: −0.178–0.174) and was contained within the non-inferiority margin (Fig. 2). Three years following treatment, the mean difference again showed non-inferiority of A-CXL.
Keratometry and pachymetry
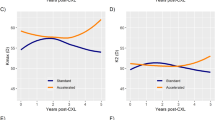
Kmax decreased by a mean of 0.98 ± 5.56 dioptres at 1 year following S-CXL and by 1.48 ± 8.4 dioptres following A-CXL. The mean difference between modalities at 1 year was −0.5 dioptre (95% CI: −3.3–2.3 dioptres) with 95% CI exceeding the pre-determined non-inferiority margin of ±1 dioptre (Fig. 2). At 3 years, the difference was 0.36 dioptres (95% CI: −0.66–1.38 dioptres), again exceeding the non-inferiority margin. In both treatment groups the Kmax was reduced overall, however non inferiority cannot be claimed, mainly due to the large spread in the results (Fig. 2). At 3 years reductions in Kmax were 56.3 ± 5.0 to 55.1 ± 5.2 in the S-CXL group (p = 0.002) and 54.9 ± 4.7 to 54.2 ± 4.9 (p = 0.015) in the A-CXL groups. There were no differences between the two treatment groups in terms of regression, stabilization, or progression (p > 0.302 for all comparisons). In addition, no significant differences were seen among the two groups in their average anterior and posterior, Ksteep, Kflat and astigmatism, three years following CXL, Table 3.
Thinnest pachymetry at 1-year decreased by 26.8 ± 40.7 µm following S-CXL and by 10.2 ± 13.4 µm following A-CXL. The mean difference between treatment types was 16.6 µm (95% CI: 3.16–30.05), showing A-CXL’s non-inferiority (Fig. 2). Although A-CXL had considerably lower loss of pachymetry at 1-year, a favourable outcome, the variation was large and so only non-inferiority could be proven, this is illustrated in Fig. 2. For thinnest pachymetry at 3-years, a mean difference of 3.84 µm was seen (95% CI: −6.57–14.27), demonstrating non-inferiority of A-CXL (Fig. 2).
Corneal astigmatism at 1-year following S-CXL decreased by a mean of 0.497 ± 2.8 dioptres and by 0.4122 ± 1.45 dioptres following A-CXL. The mean difference between treatment types was 0.055 dioptres (95% CI: −0.925–1.03 dioptres). As the higher end of the margin (1.03 dioptres) just exceeds the non-inferiority margin of ±1 dioptre, non-inferiority compared to S-CXL cannot be determined. After 3 years, the mean difference was −0.44 dioptres (95% CI: −1.06–0.17 dioptres), showing non-inferiority of A-CXL as seen in Fig. 2.
Discussion
This study compared the traditional protocol for CXL (S-CXL “Dresden”) to an accelerated protocol (A-CXL) that uses higher intensity for a shorter duration among 93 paediatric patients with KC. Following both treatments, an improvement in BSCVA and UCVA was seen, and a reduction in Kmax and astigmatism was noted, yet thinnest pachymetry continued to decrease. Accelerated CXL was non-inferior to standard CXL in terms of best-corrected and uncorrected visual acuity and thinnest pachymetry at one- and three-year following treatment and in terms of astigmatism at 3 years. For Kmax and astigmatism at 1-year, non-inferiority could not be concluded.
A-CXL is a more suitable treatment for the paediatric population as it reduces the time needed for the patient to lie still, which is challenging at a younger age. Another benefit of a shorter protocol is reduced corneal dehydration and intraoperative thinning, leading to a safer treatment [15]. In the adult population, the safety, and efficacy of A-CXL have been established; A recent meta-analysis evaluating 22 studies and 1158 eyes (S-CXL: 577; A-CXL: 581 eyes) showed similar effects of both treatment in terms of visual acuity, refraction, keratometry, pachymetry and biomechanical properties post-CXL [16]. However, among paediatric patients, less data is available.
The efficacy of CXL (standard treatment) is well established in patients under the age of 18. It was shown in multiple previous studies that CXL can halt keratoconus progression in that population [17, 18]. In the paediatric population, fewer studies compared the S-CXL and A-CXL. Baenninger et al. and Turhan et al. showed that accelerated treatment appears to be as effective as standard protocol in stabilizing keratoconus progression in paediatric patients [10, 11]. Their follow-up periods were limited to one and two years, respectively. Our findings correlate with these previous studies showing the same efficacy for the two protocols in a longer follow-up time. In our study, the S-CXL and the A-CXL experienced a significant improvement in visual function three years after surgery. Turhan et al. found significant improvement of UCVA two years after CXL in the S-CXL group but no significant change in the A-CXL group [10]. However, other previous studies did show UCVA and BSCVA improvement in paediatric patients treated with the accelerated protocol [13, 19]. We also found that eyes in the A-CXL experienced a faster improvement of BSCVA compared to S-CXL.
While both treatment groups resulted in Kmax reduction, we could not confirm non-inferiority for the A-CXL. This observation was repeated by a meta-analysis of 12 trials showing that S-CXL appeared to flatten the Kmax significantly more than A-CXL (Standardized mean difference: 0.32; 95% CI 0.16−0.48) [20]. It seems that the increased effect of S-CXL on Kmax becomes more pronounced with follow-up time, as Kmax values were similar at 1, 3 and 6 months but became significant at 6 and 18 months (meta-analysis Shajari et al. comparing S-CXL (n = 577) A-CXL (n = 581)) [16]. In addition, a study of an even shorter A-CXL protocol (5 min of 18.0 mW/cm2) showed stable Kmax compared to a 3.21 ± 3.79D decreased by the S-CXL protocol, at five year-follow-up, p = 0.02 [20]. However, this differential effect on Kmax did not clinically translate to an improved visual acuity in our data nor the recent mata-analysis studies [16, 21].
Progression, an increase of Kmax by more than one dioptre, was the same in both groups (S-CXL: 11.11% and A-CXL: 12.82%, p = 1.0). A similar rate Kmax increase (>1D) post-CXL in paediatric patients were reported by both Turhan et al. (S-CXL: 7.8% and A-CXL: 18.2%, p = 0.44) and Sarac et al. (S-CXL: 16.3% and A-CXL: 13.1%, p = 0.754 at 2-years follow-up [8, 10].
This study’s limitations include its retrospective nature, the lack of randomization, and the fact that treatments were given at two different centres. We mitigate this possible bias by choosing centres with the same corneal tomographer, the same definitions for progression, and the same CXL protocols, by comparing baseline variables, and by comparing each group to itself and only comparing the influence of treatment between centres. Despite this, confounders could naturally still exist, some that cannot be measured or accounted for. Some differences in baseline variables exist, none apart from BCVA were statistically significant. As for BCVA the baseline values were significantly different and a possible influence on the results cannot be ruled out. Results should be interpreted accordingly. We included in the study only eyes with Kmax <69 D and Pachymetry >400 micron hence our results and conclusions can only be implemented on that group of patients.
To conclude, among paediatric patients with keratoconus followed for three years, A-CXL was non-inferior to S-CXL in terms of best-corrected and uncorrected visual acuity, thinnest pachymetry, and astigmatism. An accelerated protocol may be more beneficial in patient comfort and more suited to the paediatric population.
Summary
What was known before
-
Standard corneal collagen cross-linking (S-CXL) is an effective treatment to arrest Keratoconus (KC) progression in children
-
Limited data is available assessing the outcomes of accelerated CXL (A-CXL) in paediatric populations
What this study adds
-
Paediatric patients followed for three years after A-CXL showed improved visual function, reduced corneal astigmatism and Kmax, and decreased thinnest corneal thickness.
-
A-CXL was non-inferior to S-CXL at three years in terms of best-corrected and uncorrected visual acuity, thinnest pachymetry, and astigmatism.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Andreanos KD, Hashemi K, Petrelli M, Droutsas K, Georgalas I, Kymionis GD. Keratoconus Treatment Algorithm. Ophthalmol Ther. 2017;6:245–62. https://doi.org/10.1007/s40123-017-0099-1.
Torres Netto EA, Al-Otaibi WM, Hafezi NL, Kling S, Al-Farhan HM, Randleman JB, et al. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br J Ophthalmol. 2018;102:1436–41. https://doi.org/10.1136/bjophthalmol-2017-311391.
Olivo-Payne A, Abdala-Figuerola A, Hernandez-Bogantes E, Pedro-Aguilar L, Chan E, Godefrooij D. Optimal management of pediatric keratoconus: challenges and solutions. Clin Ophthalmol. 2019;13:1183–91. https://doi.org/10.2147/OPTH.S183347.
Sarac O, Caglayan M, Cakmak HB, Cagil N. Factors influencing progression of keratoconus 2 years after corneal collagen cross-linking in pediatric patients. Cornea. 2016;35:1503–7. https://doi.org/10.1097/ICO.0000000000001051.
Duncan JK, Belin MW, Borgstrom M. Assessing progression of keratoconus: novel tomographic determinants. Eye Vis (Lond). 2016;3:6 https://doi.org/10.1186/s40662-016-0038-6.
Soeters N, van der Valk R, Tahzib NG. Corneal cross-linking for treatment of progressive keratoconus in various age groups. J Refract Surg. 2014;30:454–60.
El Rami H, Chelala E, Dirani A, Fadlallah A, Fakhoury H, Cherfan C, et al. An update on the safety and efficacy of corneal collagen cross-linking in pediatric Keratoconus. Biomed Res Int. 2015;2015:257927. https://doi.org/10.1155/2015/257927.
Salah Y, Omar K, Sherif A, Azzam S. Study of demarcation line depth in transepithelial versus epithelium-off accelerated cross-linking (AXL) in Keratoconus. J Ophthalmol. 2019;2019:1–4. https://doi.org/10.1155/2019/3904565.
Sarac O, Caglayan M, Uysal BS, Uzel AGT, Tanriverdi B, Cagil N. Accelerated versus standard corneal collagen cross-linking in pediatric keratoconus patients: 24 months follow-up results. Cont Lens Anterior Eye. 2018;41:442–7. https://doi.org/10.1016/j.clae.2018.06.001.
Turhan SA, Yargi B, Toker E. Efficacy of conventional versus accelerated corneal cross-linking in pediatric Keratoconus: two-year outcomes. J Refract Surg. 2020;36:265–9. https://doi.org/10.3928/1081597X-20200302-01.
Baenninger PB, Bachmann LM, Wienecke L, Thiel MA, Kaufmann C. Pediatric corneal cross-linking: comparison of visual and topographic outcomes between conventional and accelerated treatment. Am J Ophthalmol. 2017;183:11–16. https://doi.org/10.1016/j.ajo.2017.08.015.
Godefrooij DA, Roohé SL, Soeters N, Wisse RPL. The independent effect of various cross-linking treatment modalities on treatment effectiveness in Keratoconus. Cornea. 2020;39:63–70.
Shetty R, Nagaraja H, Jayadev C, Pahuja NK, Kurian Kummelil M, Nuijts RMMA. Accelerated corneal collagen cross-linking in pediatric patients: two-year follow-up results. Biomed Res Int. 2014;2014:894095. https://doi.org/10.1155/2014/894095.
Rosser DA, Cousens SN, Murdoch IE, Fitzke FW, Laidlaw DAH. How sensitive to clinical change are ETDRS logMAR visual acuity measurements? Invest Ophthalmol Vis Sci. 2003;44:3278. https://doi.org/10.1167/iovs.02-1100.
Holopainen JM, Krootila K. Transient corneal thinning in eyes undergoing corneal cross-linking. Am J Ophthalmol. 2011;152:533–6. https://doi.org/10.1016/j.ajo.2011.03.023.
Shajari M, Kolb CM, Agha B, Steinwender G, Müller M, Herrmann E, et al. Comparison of standard and accelerated corneal cross-linking for the treatment of keratoconus: a meta-analysis. Acta Ophthalmol. 2019;97:e22–e35. https://doi.org/10.1111/aos.13814.
Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A, et al. Corneal collagen cross-linking with riboflavin and Ultraviolet A light for pediatric Keratoconus: Ten-year results. Cornea. 2018;37:560–6. https://doi.org/10.1097/ICO.0000000000001505.
Li J, Ji P, Lin X. Efficacy of corneal collagen cross-linking for treatment of Keratoconus: a meta-analysis of randomized controlled trials. PLoS ONE. 2015;10:e0127079 https://doi.org/10.1371/journal.pone.0127079.
Ulusoy DM, Göktaş E, Duru N, Özköse A, Ataş M, Yuvacı İ, et al. Accelerated corneal crosslinking for treatment of progressive keratoconus in pediatric patients. Eur J Ophthalmol. 2017;27:319–25. https://doi.org/10.5301/ejo.5000848.
Kato N, Negishi K, Sakai C, Toda I, Ide T, Torii H, et al. Five-year outcomes of corneal cross-linking for Keratoconus: comparison between conventional and accelerated procedures. Cornea. 2020;39:e1–e1. https://doi.org/10.1097/ICO.0000000000002174.
Wen D, Li Q, Song B, Tu R, Wang Q, O’Brart DPS, et al. Comparison of standard versus accelerated corneal collagen cross-linking for Keratoconus: a meta-analysis. Invest Ophthalmol Vis Sci. 2018;59:3920. https://doi.org/10.1167/iovs.18-24656.
Author information
Authors and Affiliations
Contributions
AEL was responsible for designing the protocol, interpreting results and led the writing of the manuscript. AA contributed to result interpretation and the writing of the final manuscript. SH was responsible for extracting the data. IH was responsible for analyzing data and interpreting results. BDS was responsible for analyzing data, interpreting results and contributed to the writing of the final manuscript. BK contributed to extracting data, result interpretation and the writing of the final manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Einan-Lifshitz, A., Achiron, A., Hed, S. et al. Three-year follow-up of accelerated versus standard corneal cross-linking in paediatric Keratoconus. Eye 37, 1219–1224 (2023). https://doi.org/10.1038/s41433-022-02093-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02093-4