Abstract
Background
To report the long-term outcomes of round autologous anterior lens capsules (ALCs) cut by a femtosecond laser (FSL) and transplanted onto refractory macular holes (MHs) in a prospective interventional study.
Methods
Three eyes of three patients were included for persistent MH after reattached rhegmatogenous retinal detachment (RRD) (n = 2) or RRD recurrence by persistent MH (n = 1), in a university hospital. A 6 mm diameter ALC disc was carefully extracted during FSL-assisted lens extraction, stained with 0.06% trypan blue, decellularised, transplanted using a catheter and unfolded over the MH. Gas or silicone-oil tamponade was used. At 1 year, the main criterion was anatomic success, defined as complete MH closure. Secondary criteria were changes in best corrected visual acuity (BCVA), ellipsoid zone (EZ) and external limiting membrane (ELM) defects, complications.
Results
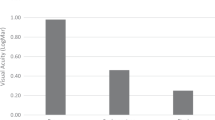
Baseline data were: minimum and maximum diameters, respectively 887, 1079 and 1180 μm; 1260, 1213 and 1350 μm; central posterior staphyloma in two highly myopic eyes; number of prior surgeries 2 ± 1. At 1 year, the three MHs were closed with stable transplanted ALCs. Distant BCVA improved respectively from 3.0, 0.8, 3.0 to 1.0, 0.2, 0.7 logMAR, i.e. all eyes achieved ≥0.3 logMAR improvement. All patients had decreased EZ and ELM defects, without reaching normal profile. No adverse event occurred.
Conclusions
FSL-cut ALC helps standardise this challenging surgery: it prevents from tears and facilitates manipulation, so that the ALC disc is perfectly transparent and biocompatible, with a large MH overlap. One-year follow-up highlighted that this technique helps safely close refractory MHs with satisfactory visual recovery.
Similar content being viewed by others
Introduction
There is no codified approach to treating refractory full-thickness macular holes (MH). The options are: ab externo [1, 2], or ab interno more recently, mostly using adjuvants. Four tissues can be transplanted into or over the MH: autologous internal limiting membrane flap [3, 4] (ILM), autologous lens capsular flap transplant [5] (LCFT), autologous retinal transplant [6] (ART), amniotic membrane [7, 8] (AM). The use of autologous platelet concentrate (APC) [9] has also been proposed. More marginally, to reduce the size of the absolute central scotoma, some authors have proposed perifoveolar laser photocoagulation [10], tapping the MH edges [11], arcuate retinotomy on the MH margin [12] and macular detachment [13, 14]. Technique selection depends on the anatomical status of the operated eye and on the surgeon’s experience: only a recent review [15] suggests that the most effective adjuvants are AM, LCFT and APC, providing better visual recovery than free ILM flap. Refractory MH treatment remains a complex procedure for multioperated patients and any potential refinement merits consideration. We have recently reported [16] the transplantation of a large disc of lyophilised AM (LAM) stained with trypan blue (TB) to facilitate its placement over the MH as an overlay [17].
For the same purpose, we report here the transplantation of a femtosecond laser (FSL)-cut and blue-stained anterior lens capsule disc.
Materials and methods
Study design
We conducted a monocentric prospective interventional consecutive case series, in a university hospital (Saint-Etienne, France) with at least 12-months’ follow-up. All patients were informed of the nature and intent of the study, and their written consent was collected. The study was approved by our local Institutional Review Board (IORG0007394, IRBN172019/CHUSTE) and conducted in accordance with the tenets of the Declaration of Helsinki.
Inclusion criteria were cataract and persistent MH after at least one prior surgery involving extended ILM removal and intraocular tamponade. Exclusion criteria were patients who had any ocular history of diabetic retinopathy, vascular occlusion, retinal neovascularization, inflammatory disease and trauma. We included three eyes of three patients, followed up from December 2019 to June 2021. Two patients had persistent MH after RRD reattachment: one with a peripheral tear, the other after MH-induced RRD in a highly myopic (HM) eye. The third had RRD by persistent MH with grade C proliferative vitreoretinopathy (PVR) located inferiorly in an HM eye: in this patient’s previous surgery, we were unable to unfold a disc of LAM due to poor visualisation [16].
Pre-transplantation examination comprised: distant and near best-corrected visual acuity (BCVA) measured with Snellen and Parinaud charts respectively, metamorphopsia assessed by Amsler gird charts [18], Goldmann applanation tonometry, fundus dilated exam, spectral-domain OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany), and when possible, fundus retinography and autofluorescence imaging (CR2-AF; Canon, Tokyo, Japan). The MH was measured by one experienced observer (T.G.) per the gold standard of the International Vitreomacular Traction Study group [19]: MH size was defined anatomically by OCT-based measurement of minimum hole width or inner opening diameter, using the inbuilt OCT calliper tool (Heidelberg Eye Explorer; Heidelberg Engineering). The maximum or basal MH diameter was also measured at the level of retinal pigment epithelium (RPE).
The main criterion at 1 year was anatomic success, defined as complete MH closure [20]. Secondary criteria were: 1/Changes in BCVA defined as an increase or a decrease of at least 0.3 logarithm of the minimal angle resolution (logMAR) units (equivalent to a 15-letter change on the Early Treatment of Diabetic Retinopathy Study chart); 2/Changes in ellipsoid zone (EZ) and external limiting membrane (ELM) defects measured by OCT. These were assessed using the inbuilt OCT calliper tool (Heidelberg Eye Explorer, Heidelberg Engineering GmbH); 3/Potential surgical complications; 4/ALC disc status: bleaching, integration or disappearance. All patients underwent examination at day (D)1, 15, month (M)1, M3, then every 3 months. Interim visits were as needed.
Surgical technique
Figure 1 shows the preparation and insertion steps images of the FSL-cut ALC disc transplantation. FSL-assisted cataract surgery was performed using a Z8 laser (Ziemer, Biel, Switzerland). We used a 6 mm diameter capsulorhexis. After staining by 0.06% TB (VisionBlue; DORC International, Zuidland, Netherlands), the intact ALC disc was extracted under a viscodispersive substance (Viscoat; Alcon, Forth Worth, TX, USA), then decellularised by gentle scrapping with a microsponge in sterile water. We stored the disc in balanced salt solution (BSS) where it rolled up. A pars plana vitrectomy was performed using a three-port 23-gauge(G) system (Constellation; Alcon). Before transplantation, we left a small volume of BSS over the macular area: only a partial fluid–air exchange was affected before ALC placement. We loaded the rolled-up ALC in a 24 G catheter (Insyte; BD, Pont de Claix, France) by direct aspiration, to avoid grasping the disc with a forceps to avoid tearing. We injected the ALC at the fluid-air interface. Fluid-air exchange was then completed to accurately position and fully unfold the ALC over the macula. To help stabilise the ALC disc, a drop of Viscoat was deposited over it. Tamponade was then carefully injected using gas (C2F6 18%; Arceole; Arcad, Toulouse, France) (n = 2) or heavy silicone-oil (Densiron 68; Fluoron, Ulm, Germany) (n = 1). Patients adopted the usual position for each tamponade. Heavy silicone oil was chosen for one patient and not removed, given their previous complicated surgical history and grade-C inferior PVR.
A The anterior segment just after femtolaser procedure by Z8 Ziemer (lens fragmentation, capsulotomy, clear corneal incisions). Air bubbles are released, with clear capsulotomy detached with anterior cortex, stained by trypan blue. B The anterior capsule was removed carefully with rhexis forceps under viscodispersive solution (Viscoat, Alcon). C The blue-stained capsule was prepared with decellularisation in sterile water, and gently scraped by a microsponge. D The prepared ALC was carefully aspirated into a catheter (24-gauge) using BSS, then inserted through sclerotomy E under wide field BIOM into the vitreous cavity at the fluid–air interface (F). A small volume of BSS was left in the macular area. Fluid-air exchange was then completed to accurately position and fully unfold the ALC in the macular area: a silicone tip backflush cannula was used to centre the ALC over the MH and ensure it adhered properly to the underlying inner retina, with only slight manipulation.
Results
The Table 1 gives the patients’ preoperative demographic and anatomic characteristics. At 1 year, anatomic success was achieved in three eyes (100%), with closure of MHs of type 1B (n = 2), type 2 A (n = 1) [20]. The RRD was reattached without recurrence. Distant and near BCVA improved in three eyes (100%): preoperative distant BCVA scores were 3.0, 0.8, and 3.0 and reached respectively 1.0, 0.2, and 0.7 logMAR at 1 year; preoperative near BCVA scores were 3.0, 0.8 and 3.0 and reached respectively 0.9, 0.1 and 0.8 logMAR at 1 year. Metamorphopsia decreased at M1, and was unchanged thereafter. Preoperative EZ defects lengths were 1297, 1285 and 1560 and respectively decreased to 742, 604 and 790 μm at 1 year. Preoperative ELM defects lengths were 1158, 1110 1450 and respectively decreased to 625, 496, 654 μm at 1 year. The ALC discs were transparent at D1 and remained visible with OCT at 1 year. Two ALC remained stable on the retinal surface. The third (type 2A closure) appeared like interposed filling tissue. No adverse ALC-related event occurred.
Figure 2 showed a case with previous failure of LAM transplantation, and Fig. 3 a case without concomitant RRD during follow-up.
A Baseline optical coherence tomography (OCT) and infra-red imaging showing a very large (minimum diameter 887μm) residual macular hole (MH), with a double roll of lyophilised amniotic membrane (LAM, Visio Amtrix, TBF, France) adhering to the edge of the MH, 6 months (M) after LAM transplantation. Then, an MH-induced rhegmatogenous retinal detachment occurred at M10. Preoperative distant visual acuity (VA) was 20/20000 (3.0 logarithm of the minimal angle resolution (logMAR)) on this highly myopic eye. B At M1 on comparative B-scan OCT (green dashed line), the ALC transplant was visible as an epiretinal thin dashed hyperreflective line with a slightly sharp interpapillomacular edge. A type 1B closure occurred. The fundus image shows the reflection of the silicone interface, but not the transparent ALC. C At M18, the ALC disc remained visible and no dislocation occurred on comparative B-scan OCT (green dashed line). Intraretinal microcystic spaces were also visible (theses appeared at M6 and remained stable thereafter) with a fairly satisfactory foveal profile. Distant VA improved to 20/200 (1.0 logMAR). The fundus image remained stable. The silicone-oil was not removed, given the subject’s complicated surgical history.
A Baseline optical coherence tomography (OCT) and infra-red imaging showing a very large macular hole, with minimum diameter 1079 μm & maximum diameter 1213 μm. Preoperative distant visual acuity (VA) was 20/125 (0.8 logarithm of the minimal angle resolution (logMAR)), metamorphopsia were present. B At month (M)1 on comparative B-scan OCT (green dashed line), the ALC transplant was visible as an epiretinal hyperreflective line; type 1B complete closure with microcystic spaces occurred. The fundus image showed a normal appearance. C At M12 on comparative B-scan OCT (green dashed line), the retinal layers had undergone further restoration, which was correlated with distant VA improvement (20/32, 0.2 logMAR) and decreased metamorphopsia. The ALC transplant seemed slightly bent. The fundus image remained stable and the ALC invisible.
Discussion
FSL-cut ALC discs, transplanted as epiretinal patches over refractory MHs, achieved anatomic closure and notable restoration of EZ and ELM without reaching normal profile [20] at 1 year. Visual improvement and decreased metamorphopsia occurred in all three patients. These findings are encouraging as all patients had risk factors for poor visual outcome [21]; persistent MH, MH-associated RRD in an HM eye, very large baseline minimum MH diameter and posterior staphyloma.
We chose ALCs for eyes with cataract requiring a combined procedure, as no other alternative seemed viable: two HM patients had posterior staphyloma, making it difficult to peel ILM remnants outside the macula [4]; the MH-associated RRD had extensive PVR; LAM had failed in one case [16]; ART seemed too invasive. Besides, a fully biocompatible and transparent ALC could give better visual results than AM [15]. We combined the advantages of ALC with the epiretinal position (i.e. overlay [17]), as we previously discussed for LAM [16]: 1/The overlay could play the same role as an inverted ILM flap [22], but would be larger, easier to position, and more stable. Like a biological bandage, it could act as a scaffold to promote healing, with centripetal migration of cells, stimulation of macrophage-like cells facilitating MH closure, and a closure mechanism more physiological than that of the subretinal position [23]; but if complete closure proved impossible, it could also act as a patch and prevent MH-induced RRD. We hoped to obtain excellent functional results by analogy with those already obtained for an ILM used as an epiretinal inverted flap versus insertion into the MH [24]: epiretinal position resulted in significantly better recovery of photoreceptor layers (fewer ELM and EZ defects), and thus visual recovery; 2/The overlay better respects the organisation of all retinal layers, preventing induction of foveal gliosis by interposition of exogenous tissue (AM) placed in the subretinal space (i.e. inlay [17]), which must be integrated between the MH edges; 3/It is safer not to manipulate or massage the MH edges, so as not to worsen the RPE and neuroretina injuries, particularly during disc insertion [25]; 4/The overlay could prevent the parafoveal atrophy described after retraction of AM used as an inlay [26]; 5/Even considering the time taken to fully unfold the LAM as an overlay, operating time is shorter than with inlay, thus reducing phototoxicity [27]; 6/If an adverse event occurs, the LAM can be removed, so this new surgical technique is reversible.
Chen et al. pioneered LCFT on refractory MHs [5], using ALCs obtained by manual continuous curvilinear capsulorhexis (CCC). Variations were subsequently described using ALC or posterior lens capsule (PLC) [28,29,30] extracted manually. Using the ALC presents several advantages [5, 28] versus PLC: it is stiffer and easier to manipulate, and larger flaps can be cut to fill or cover the MH. The PLC may also be fibrotic and/or colonised by lens epithelial cells, which are difficult to extract and may destabilise the intraocular lens in complex eyes.
Contrary to CCC, only FSL (or the less-common selective laser capsulotomy or thermal capsulotomy devices) can standardise capsule extraction to cut an intact, large diameter ALC disc without tearing. The large surface of the disc made it easier to manipulate and to position over the MH, while avoiding potential additional trauma to the central RPE and allowing a potential slight postoperative off-centre shift with no consequences. The FSL is an expensive device, not available in all centres. However, it can be a very valuable option rather than CCC, to prepare an optimised ALC transplant, ensuring best anatomic and functional postoperative results.
Staining every disc with TB seems to be crucial for manipulations, mainly because the ALC is fully transparent, unlike LAM [16]. TB is not toxic for the RPE [31], unlike the indocyanine green used in other series [5, 28,29,30].
This technique is almost ‘no touch’: the ALC is inserted with a catheter to prevent direct manipulation and tears, as it is slightly more brittle than ILM or LAM. We used a viscodispersive substance to stabilise the unfolded ALC, rather than additional direct contact with a silicon tip or APC [28,29,30] which can sometimes leak into the subretinal space and cause additional damage [32]. In their series, Peng et al. [28, 30] justified APC as an adjuvant to reduce toxicity associated with indocyanine green staining of the ALC. We thus applied a viscodispersive substance to minimise the possibility of ALC displacement after surgical repair, avoiding the risks of APC (potential toxicity for photoreceptors, and infectious risk).
Removal of a persistent ALC transplant was not envisaged as the gain was uncertain [33]: no patient was not subjectively dissatisfied and there was a theoretical risk of MH recurrence and of functional or anatomic deterioration through subsequent surgery on multioperated eyes.
The strengths of this study were: a prospective follow-up of at least 1 year; homogeneous baseline prognosis; and the use of standardised definitions, surgery and imaging. Its Limitations: this was a monocentric, non-controlled, non-randomised case series. Larger numbers with longer follow-up are needed.
A transplanted of FSL-cut ALC overlay can help safely close refractory MHs in eyes with cataract requiring a combined procedure, and provides satisfactory visual recovery after a 1-year follow-up. The FSL standardises ALC preparation by delivering a large intact disc, and injection through a catheter facilitates this challenging surgical procedure.
Summary table
What was known before
-
Autologous anterior or posterior lens capsule extracted manually (curvilinear capsulorhexis) helps close refractory macular holes when transplanted in subretinal position. Anterior lens capsule use is easier to extract and manipulate, thus safer than posterior lens capsule use.
What this study adds
-
Cutting autologous anterior lens capsule by femtosecond laser helps standardise a challenging surgery to close refractory macular holes. Femtosecond laser prevents from anterior capsule tears and facilitates handling to safely unfold the disc with perfect transparency, biocompatibility, and large overlapping. Thanks to a femtosecond laser-cut autologous anterior lens capsule disc transplanted in epiretinal position, complete closure of refractory macular holes was achieved with satisfactory visual recovery.
Data availability
The datasets used and/or analysed during the current study are available from TG or GT on reasonable request.
References
Sasoh M, Yoshida S, Ito Y, Matsui K, Osawa S, Uji Y. Macular buckling for retinal detachment due to macular hole in highly myopic eyes with posterior staphyloma. Retina. 2000;20:445–9.
Kono T, Takesue Y, Shiga S. Scleral resection technique combined with vitrectomy for a macular hole retinal detachment in highly myopic eyes. Ophthalmol J Int. 2006;220:159–63.
Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–25.
Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157:861–869 e861.
Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36:163–70.
Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134:229–30.
Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39:S95–S103. Suppl 1
Moharram HM, Moustafa MT, Mortada HA, Abdelkader MF. Use of epimacular amniotic membrane graft in cases of recurrent retinal detachment due to failure of myopic macular hole closure. Ophthalmic Surg Lasers Imaging Retin. 2020;51:101–8.
Gaudric A, Massin P, Paques M, Santiago PY, Guez JE, Le Gargasson JF, et al. Autologous platelet concentrate for the treatment of full-thickness macular holes. Graefe’s Arch Clin Exp Ophthalmol. 1995;233:549–54.
Harris MJ, de Bustros S, Michels RG. Treatment of retinal detachments due to macular holes. Retina. 1984;4:144–7.
Kumar A, Tinwala SI, Gogia V, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia Pac J Ophthalmol. 2013;2:305–9.
Charles S, Randolph JC, Neekhra A, Salisbury CD, Littlejohn N, Calzada JI. Arcuate retinotomy for the repair of large macular holes. Ophthalmic Surg Lasers Imaging Retin. 2013;44:69–72.
Szigiato AA, Gilani F, Walsh MK, Mandelcorn ED, Muni RH. Induction of macular detachment for the treatment of persistent or recurrent idiopathic macular holes. Retina. 2016;36:1694–8.
Fotis K, Alexander P, Sax J, Reddie I, Kang CY, Chandra A. Macular detachment for the treatment of persistent full-thickness macular holes. Retina. 2019;39:S104–S107. Suppl 1
Frisina R, Gius I, Tozzi L, Midena E. Refractory full thickness macular hole: current surgical management. Eye. (2021). https://doi.org/10.1038/s41433-020-01330-y
Garcin T, Gain P, Thuret G. Epiretinal large disc of blue-stained lyophilized amniotic membrane to treat complex macular holes: a 1-year follow-up. Acta Ophthalmol. 2022;100:e598–e608.
Letko E, Stechschulte SU, Kenyon KR, Sadeq N, Romero TR, Samson CM, et al. Amniotic membrane inlay and overlay grafting for corneal epithelial defects and stromal ulcers. Arch Ophthalmol. 2001;119:659–63.
Schuchard RA. Validity and interpretation of Amsler grid reports. Arch Ophthalmol. 1993;111:776–80.
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–9.
Rossi T, Bacherini D, Caporossi T, Telani S, Iannetta D, Rizzo S, et al. Macular hole closure patterns: an updated classification. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:2629–38.
Alkabes M, Pichi F, Nucci P, Massaro D, Dutra Medeiros M, Corcostegui B, et al. Anatomical and visual outcomes in high myopic macular hole (HM-MH) without retinal detachment: a review. Graefe’s Arch Clin Exp Ophthalmol. 2014;252:191–9.
Boninska K, Nawrocki J, Michalewska Z. Mechanism of “Flap Closure” after the inverted internal limiting membrane flap technique. Retina. 2018;38:2184–9.
Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23:421–4.
Park JH, Lee SM, Park SW, Lee JE, Byon IS. Comparative analysis of large macular hole surgery using an internal limiting membrane insertion versus inverted flap technique. Br J Ophthalmol. 2019;103:245–50.
Caporossi T, Tartaro R, Giansanti F, Rizzo S. The amniotic membrane for retinal pathologies. Insights on the surgical techniques. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:1347–9.
Tsai D, Huang Y, Chen S. Parafoveal atrophy after human amniotic membrane graft for macular hole in patients with high myopia. Br J Ophthalmol. 2021;105:1002–10.
van den Biesen PR, Berenschot T, Verdaasdonk RM, van Weelden H, van Norren D. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol. 2000;84:1372–5.
Peng J, Chen C, Jin H, Zhang H, Zhao P. Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina. 2018;38:2177–83.
Chen YC, Yang CM, Chen SN. Lens capsular flap in the management of posterior retinal hole associated retinal detachment in high myopic eyes with previous internal limiting membrane peeling: 3 case reports. Medicines. 2019;98:e16422.
Peng J, Chen C, Zhang H, Zhang L, Liu J, Ren J, et al. Long-term surgical outcomes of lens capsular flap transplantation in the management of refractory macular hole. Retina. 2021;41:726–34.
Farah ME, Maia M, Penha FM, Rodrigues EB. The use of vital dyes during vitreoretinal surgery—chromovitrectomy. Dev Ophthalmol. 2016;55:365–75.
Benner JD, Hay A, Landers MB 3rd, Hjelmeland LM, Morse LS. Fibrinolytic-assisted removal of experimental subretinal hemorrhage within seven days reduces outer retinal degeneration. Ophthalmology. 1994;101:672–81.
Reid GA, McDonagh N, Wright DM, Yek JTO, Essex RW, Lois N. First failed macular hole surgery or reopening of a previously closed hole: do we gain by reoperating?—A systematic review and meta-analysis. Retina. 2020;40:1–15.
Author information
Authors and Affiliations
Contributions
TG conceived and designed the study, collected the data, analysed the data, and wrote the manuscript. PG supervised the writing and editing of the manuscript. GT designed the study, analysed the data and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors of this manuscript have competing interests to disclose as specified by Eye. PG and GT are consultants for Laboratoires Théa, Quantel Medical, Sincler, Keranova and Acusurgical (none related to this study). PG and GT have patented a method for preparing an allograft or xenograft material from a crystalline lens capsule (PCT WO2021013893). TG declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcin, T., Gain, P. & Thuret, G. Femtosecond laser-cut autologous anterior lens capsule transplantation to treat refractory macular holes. Eye 37, 1073–1079 (2023). https://doi.org/10.1038/s41433-022-02062-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02062-x