Abstract
Background
To evaluate the influence of age on the clinical characteristics of primary rhegmatogenous retinal detachments (RRD).
Methods
We conducted a retrospective review of a prospectively collected dataset. Data regarding adult patients (aged 16–100 years) who had undergone primary RRD repair, were extracted from two online databases. Baseline demographics, preoperative clinical characteristics and surgical management details were collected. Age-based groups (16–30, 30–39, 40–49, 50–59, 60–69, 70–79, ≥80) were compared using univariate analysis, with multivariate testing for interaction of age with sex, laterality and pseudophakia.
Results
In total, 8,133 eyes were analysed, of which the majority (59%) were in the 50–69 age-range peaking at 60, with a male predominance (64%). Myopia was significantly more frequent in patients aged <50 years. The presence of posterior vitreous detachment increased up to 50 years, then remained >95%. Foveal involvement, grade C proliferative vitreoretinopathy, total RD and greater RD extent were more common and progressively increased after 60 years, with worsening visual acuity. Isolated superior RRDs became more prevalent with age reaching a plateau in the age-range 50–69, before reducing again; conversely, isolated inferior RRDs were commoner in those <30, with a minimum in the 70–79 age-range. The incidence of fellow-eye RRD decreased linearly with age.
Conclusions
Age appeared a key variable in RRD phenotype influencing a wide range of RRD characteristics. The higher incidence of myopia, PVD absent and bilateral RRD in patients <40 years and the significant phenotypical differences in the under 40 and over 50 age-groups highlight that there are several discrete forms of RRD.
Similar content being viewed by others
Introduction
Rhegmatogenous retinal detachment (RRD) is the most common form of retinal detachment having an annual incidence in the general population of between 0.01–0.02% [1,2,3]. Several factors have been associated with increased risk of RRD, such as pre-existing peripheral retinal degenerations, high myopia, Caucasian or Asian ethnicity, fellow eye history, cataract surgery and age [1,2,3,4,5,6,7,8].
It is known that the incidence of RRD varies with age. In particular, two proposed peaks have been identified in the age distribution of RRD, namely the third decade of life and the interval between 55 and 69 years of age [9,10,11,12]. The former has been mainly associated with a concurrent diagnosis of myopia; [10, 11, 13] whereas the latter has been seen to correlate with a higher prevalence of posterior vitreous detachment (PVD), that has a crucial role in the pathogenesis of RRD [12]. Moreover, some evidence suggests a relationship between age and RRD features. For instance, it has been reported that the likelihood of foveal involvement increases as age increase, whilst more controversially there has been an association between age and inferior retinal breaks proposed [7, 8]. Patients aged more than 80 have also been reported to have worse single surgery anatomic success rate compared to younger patients [14, 15].
It is known that a detailed characterization of RRD in terms of phenotype plays an important role in understanding pathogenesis and in surgical planning. However, exactly how age alters the phenotype of the RRD has not been studied in a large cohort. This has particular significance with population ageing occurring in many countries [15, 16].
In the light of this, our aim was to analyse the effect of age on primary adult RRD in terms of their clinical characteristics and surgical management in a large prospectively collected database cohort.
Methods
The data for this analysis were extracted from the Britain & Eire Association of Vitreoretinal Surgeons (BEAVRS) RRD audit database and the Euretina RRD database in March 2020, including all RRDs in patients 16 years of age and older, that had undergone surgery of any type (i.e. vitrectomy, buckling, pneumatic retinopexy or combinations thereof) from May 2011 to May 2019 The two databases are based on the same methodology and inclusion/exclusion criteria for data collection. The BEAVRS database is compliant with the UK national RD dataset (https://www.rcophth.ac.uk/standardspublications-research/audit-and-data/clinical-data-sets/retinal-detach ment-data-set/). It only includes primary RRDs, and excludes RDs secondary to severe contusion, penetrating injury, vaso-proliferative disorders, inflammatory eye disease, ocular dystrophies and syndromic pediatric RD (<16 years old).
Data is entered at the end of surgery and then again after a postoperative follow-up (FU) of at least 2 months. The data collected include demographic and preoperative clinical findings such as age, sex, comorbidity, lens status, best corrected visual acuity (VA), duration of central vision loss, ocular co-pathology potentially interfering with the functional outcomes, presence or absence of PVD (assessed intraoperatively), presence and grade of vitreous haemorrhage (VH) [17], anatomical findings of RD, and a history of a fellow eye RD. Intraoperative and surgical details such as gauge of vitrectomy system, type of tamponade, type of scleral buckle (SB) if performed, modality of retinopexy, combination of cataract surgery, and any complications are recorded.
Importantly, to facilitate the data collection, a RD drawing tool is integral to the database which allows the user to record the RD distribution, extent, location, type and size of all retinal breaks, as well as features including PVR, retinoschisis, and lattice degeneration [8]. Several of the features of the RD drawing are then automatically recorded numerically including the RD extent (in clock hours and divided into quadrants), number, type and location of retinal breaks in attached and detached retina, extent of the biggest retinal break (in clock hours), location of the lowest retinal break, foveal involvement, and the presence, extent and grade of proliferative vitreoretinopathy (PVR), according to the revised Silicone Oil Study grading system [18].
Cases with incomplete documentation of age, laterality, sex, and lens status and/or incomplete retinal drawing, were excluded from the analysis.
To analyse differences in the distribution of the RD we derived a number of groups based on their location including those localized to one quadrant hemisphere only, as well as those with any involvement of the superior or inferior retina. Similarly, the position of the lowest break was categorized as being superior (10 to 2 oclock), inferior (4 to 8 oclock), and either nasal (1 to 5 oclock in right eyes and 7 to 11 oclock in left eyes) or temporal (7 to 11 oclock in right eyes and 1 to 5 oclock in left eyes).
This study followed the UK’s Data Protection Act and the declaration of Helsinki. The database does not contain any data from which the identity of a patient might be established. Internal identification is via a unique random alphanumeric code. No IRB approval and/or informed consent were therefore needed according the UK guidelines; the database being considered a service evaluation. As per study purpose, the eyes were divided according to the age-range of the patient.
Statistical analysis
R version 3.4.1 (https://www.r-project.org/) was used to perform the analyses presented. VA values were converted to the logarithm of the Minimum Angle of Resolution (logMAR), attributing the value of 1.98, 2.28, 2.70 and 3.00 to count fingers, hand movements, perception of light and no perception of light, respectively [19]. Variables were analysed based on differences between the following age groups; 16–30, 30–39, 40–49, 50–59, 60–69, 70–79, ≥80 years. Continuous variables were analysed using ANOVA and Kruskal Wallis tests, as appropriate. Associations between non-continuous variables were analysed using the chi-square test and Fisher’s exact probability. Interactions between age and the presence of pseudophakia, sex and laterality for the variables RD extent, break size, break number, VH, PVR, and the presence of inferior RD and inferior breaks were tested with logistic regression. Statistical significance was considered if p-value was less than 0.01, based on the exploratory nature of the analysis and the number of comparisons made.
Results
Data of 8,416 eyes were extracted. Of these, 283 eyes were excluded due to inappropriate entry (28) or incomplete data (255). Therefore, we analysed a total of 8,133 eyes. Eighty-four vitreoretinal surgeons contributed to the entering of the data extracted; the median of the cases per surgeon was 47 (interquartile range, IQR, 10–115).
Association of RD variables with age
The rates of the different variables analysed in the study cohort and the analysis of their association with age are showed in Table 1. The mean age was 59 years and a clear male predominance was found (63.7% of cases). The index eye was more commonly right (53.3%), phakic (72%), with PVD present (89.2%). Vitreous haemorrhage was present in 17.3%, and the superior retinal quadrants involved in 89% of cases. Foveal involvement was documented in approximately 50% of eyes. Fellow eye involvement, either concurrently or previously was recorded in 9% of cases.
A PPV was performed in the vast majority of eyes (88.7%), more commonly with short-acting gases as tamponade (53.4%).
All the variables examined were significantly associated with age (Table 1).
Comparison between age-range groups
Tables 2 and 3 show the different baseline features and surgical details by age-bands.
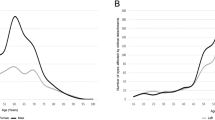
The majority of patients (59%) were in the age range 50–69 (Fig. 1A) where the male predominance was significantly more marked (p < 0.001). Myopic patients were more frequently aged <50 years (Fig. 1B). As expected, the proportion of phakic eyes with cataract and pseudophakic eyes increased linearly with age. Presenting VA worsened with age, in particular in macula off RRDs. The incidence of a PVD increased linearly up to 50 years, then reaching a plateau (>95%).
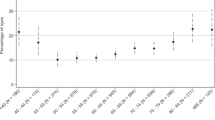
The prevalence of isolated superior and isolated inferior RRDs reached a plateau in the same age-range (50–69 years), but exhibiting two opposite trends, as the occurrence of the former increased with age, whereas the latter became less prevalent with age (Fig. 2). Isolated temporal and nasal RRD were more evenly distributed with no clear trends.
Regarding the causative retinal breaks, retinal holes and dialysis were more common in the age-range 16–40, whereas the prevalence of large retinal breaks decreased linearly with age. Similarly, the number of retinal breaks reached a maximum in the 50–69 age range, as did the presence of vitreous haemorrhage. Older patients demonstrated more severe presenting features including foveal involvement, grade C PVR, greater RD extent and concomitant choroidal detachment which all increased progressively after 60 years.
A history of fellow-eye involvement became progressively less frequent with age.
Interaction with pseudophakia, sex and laterality
None of the associations with age were confounded by the rising prevalence of pseudophakia and cataract and all associations remained significant. There was a trend towards age having a lesser effect in terms of the increase in inferior RRD in pseudophakic eyes than phakic ones although the age association of increased inferior retinal involvement at both young and older age was still significant at p = 0.005. Although reliable data on refractive status before phacoemulsification was not available, the pattern of findings of pseudophakic RD was more similar to PVD-related than myopia-related RD, consistently with previous studies [7, 8].
None of the age associations were confounded by sex or laterality, and all remained significant.
Discussion
This study is the largest study to date that has aimed to assess the influence of age on phenotype and, thus, surgical management of primary RRD. The role of age in RRD is well established; however, the analysis of a significant amount of detailed data from two databases collecting a wide range of preoperative and surgical variables, allowed us to verify previously suggested age-related features, discover new associations and also quantify effect sizes in terms of the features for which differences were found.
Consistent with data reported in the literature [2, 8,9,10,11,12], there was a predominance of males and right eyes in our study cohort, and the prevalence of primary RRD had a single peak at about 60 years old. This age distribution illustrates differences in RRD epidemiology between European and Asian populations, with the latter characterized by an additional earlier peak in RRD attributable to a higher prevalence of myopia [20, 21].
We demonstrated marked phenotypical differences between patients aged under 40, and those over 50 years of age. Young adults exhibited the highest prevalence of myopia and myopia-related RRD with absent PVD, whereas myopia appear to be become less important with age when PVD-related tears predominate. Indeed, RRD in young adults was more commonly associated with myopia, the absence of PVD, a clear crystalline lens, involvement of the inferior quadrants, inferior and round holes as the causative breaks and subretinal fibrosis. The latter is likely due to the slow progression and chronicity, typical of RRD with an attached vitreous [22]. The predominance of myopia-related RRD along with the significantly more frequent involvement of the fellow eye supports the stronger role of genetic factors in the pathogenesis of RRD at earlier age. Consistently, as demonstrated by a recent meta-analysis of genome-wide association studies, genetic variants associated with high myopia strongly contribute to the risk of RRD [23].
RRD secondary to retinal dialysis and giant retinal tears (GRT) were more prevalent as a proportion of the total in patients aged under 40 years and then decreased linearly with age, although the highest absolute number of GRT-related RRD was found in the age-range 50–59, mirroring the Scottish RRD study [24]. It is important to note that eyes with significant ocular trauma, a known risk factor for retinal dialysis and GRT [25, 26], were excluded from our database. The higher prevalence of these two distinct forms of RRD in the young age groups with exclusion of traumatic and syndromic RDs, also supports the role of predisposing genetic factors in their development [26,27,28]. In support of this, both had a higher male predominance, and GRTs had a very high fellow eye rate. Interestingly, myopia appeared to be a weaker factor for GRT and in particular dialysis RD [23].
After 50 years of age, RRD was associated more frequently with the presence of a PVD, cataract or pseudophakia, involvement of the superior quadrants with mainly superior and U-tears as causative breaks, vitreous haemorrhage, foveal involvement and grade C PVR. The rapidly rising prevalence of RRD, with the steady increase in the presence of PVD after 50 years old highlights the role of age-induced PVD as the main determining factor for the peak observed at 60. The documented predominance of U-tears located superiorly and the involvement of the superior retinal quadrants is consistent with previous evidence of a higher prevalence of superior acute PVD-related tears [29, 30]. Despite a similar rate of PVD after 50 years, the occurrence rate of concomitant VH and the number of retinal breaks peaked in the age-range 40–69 and then tailed off with age. We hypothesise that in middle-aged people an acute PVD with a more homogenous and firmly adherent vitreous predisposes to a greater number, and larger tears whilst the separation trauma is less vigorous in the presence of a liquefied vitreous gel as is the case in the eyes of older patients.
The prevalence of more advanced RRD features increased in older patients, with foveal involvement, grade C PVR, greater RD extent and concomitant choroidal detachment increasing linearly after 60 years old. Moreover, all the significant associations persisted when we tested whether the variables associated with older age were due to the rising prevalence of pseudophakia. It has been speculated that the highly liquefied vitreous of older patients could lead to a more rapid progression of RRD [15]. Consistent with these findings, baseline VA worsened with age. Co-pathologies including the presence of cataract might also explain worse vision, in line with the increased frequency of combined phaco-vitrectomy, and RRD with cataract with age. Interestingly, although the duration of central visual loss showed a U-shaped distribution being lowest in the 50–59 age group, visual acuity in foveal involving cases worsened linearly with age, probably reflecting the known preservation of acuity in shallow inferior RRD as occurred most frequently in the youngest age group. Comparing patients older than 80 years with those aged 40–79, Patel et al. [15] reported that the former exhibited more complex RRD and were more likely to undergo PPV with silicone oil tamponade. Our study confirmed a progressive increase in the use of silicone oil and long-acting gas after 50 years old, presumably related to the greater complexity of the detachment and perhaps concerns regarding compliance with posturing instructions postoperatively.
The large sample size is the major strength of this work, but we acknowledge there are many limitations. Surgeons are asked to enter consecutive cases but we cannot verify this and it therefore cannot be considered a population study. However, our sample appears to appropriately represent European populations in terms of baseline features as compared to previous national studies [2, 9]. Due to the absence of a patient identifier, the same patient could have been registered twice in case of bilateral RRD repair during the study period; however, this is likely to account for a limited proportion of cases. As per routine practice, the data entered in the database is not collected in a standardized manner with the potential for bias, however compulsory data fields and categorisation guidelines reduce variability and key missing data. Data on paediatric cases were not collected and, thus, we did not analyse primary RRD in patients younger than 16 years old. A recent cross-sectional study showed that in this age group the most common aetiologies of RRD were congenital/developmental anomalies (50%) and myopia (33.3%) [31]. Finally, detailed data were not available regarding clinical findings of the fellow eyes, as this study focused on the characterisation of the index eye. Further studies could be performed to the characterisation of the fellow eye of patients with primary RRD with age.
In conclusion, our study strongly supports the crucial influence of age on RRD phenotype. Non syndromic RRD exhibits as several different disease types with characteristic phenotypes strongly related to the age of presentation.
Summary
What was known before
-
Age influences some aspects of rhegmatogenous retinal detachment (RRD) characteristics.
What this study adds
-
RRD in <40 and >50 age-groups differ significantly supporting a completely different pathogenesis and genetic basis.
-
Increasing age alters the phenotype of RRD associated with posterior vitreous detachment in multiple different ways.
-
Composition in age should be considered in interpreting clinical studies.
Data availability
Data is freely available at https://outcomes.beavrs.org/ via ‘contact’ request and agreement to BEAVRS data access request guidelines
References
Qureshi MH, Steel DHW. Retinal detachment following cataract phacoemulsification-a review of the literature. Eye (Lond). 2020;34:616–31.
van Leeuwen R, Haarman AEG, van de Put MAJ, Klaver CCW, Los LI, Dutch Rhegmatogenous Retinal Detachment Study Group. Association of rhegmatogenous retinal detachment incidence with myopia prevalence in the Netherlands. JAMA Ophthalmol. 2021;139:85–92.
Nielsen BR, Alberti M, Bjerrum SS, la Cour M. The incidence of rhegmatogenous retinal detachment is increasing. Acta Ophthalmol. 2020;98:603–6.
Lewis H. Peripheral retinal degenerations and the risk of retinal detachment. Am J Ophthalmol. 2003;136:155–60.
Eye Disease Case-Control Study Group. Risk factors for idiopathic rhegmatogenous retinal detachment. Am J Epidemiol. 1993;137:749–57.
Chandra A, Banerjee P, Davis D, Charteris D. Ethnic variation in rhegmatogenous retinal detachments. Eye. 2015;29:803–7.
Mahroo OA, Dybowski R, Wong R, Williamson T. Characteristics of rhegmatogenous retinal detachment in pseudophakic and phakic eyes. Eye 2012;26:1114–21.
Ferrara M, Mehta A, Qureshi H, Avery P, Yorston D, Laidlaw DA, et al. Phenotype and outcomes of phakic versus pseudophakic primary rhegmatogenous retinal detachments: cataract or cataract surgery related? Am J Ophthalmol. 2020;222:318–27.
Mitry D, Charteris DG, Yorston D, Rehman Siddiqui MA, Campbell H, Murphy AL, et al. The epidemiology and socioeconomic associations of retinal detachment in Scotland: a two-year prospective population-based study. Invest Ophthalmol Vis Sci. 2010;51:4963–8.
Li X, Beijing Rhegmatogenous Retinal Detachment Study Group. Incidence and epidemiological characteristics of rhegmatogenous retinal detachment in Beijing, China. Ophthalmology. 2003;110:2413–7.
Li JQ, Welchowski T, Schmid M, Holz FG, Finger RP. Incidence of rhegmatogenous retinal detachment in Europe––a systematic review and meta-analysis. Ophthalmologica. 2019;242:81–6.
Van de Put MA, Hooymans JM, Los LI, Group DRRDS. The incidence of rhegmatogenous retinal detachment in The Netherlands. Ophthalmology. 2013;120:616–22.
Kim MS, Park SJ, Park KH, Woo SJ. Different mechanistic association of myopia with rhegmatogenous retinal detachment between young and elderly patients. BioMed Res Int. 2019;2019:5357241.
Sakamoto T, Kawano S, Kawasaki R, Hirakata A, Yamashita H, Yamamoto S, et al. Japan-Retinal Detachment Registry Report I: preoperative findings in eyes with primary retinal detachment. Jpn J Ophthalmol. 2020;64:1–12.
Patel SN, Starr MR, Obeid A, Ryan EH, Ryan C, Forbes NJ, et al. Characteristics and surgical outcomes of rhegmatogenous retinal detachment in older adults: a multicenter comparative cohort study. Retina. 2021;41:947–56.
Park SJ, Cho SC, Choi NK, Park KH, Woo SJ. Age, sex, and time-specific trends in surgical approaches for rhegmatogenous retinal detachment: a nationwide, population-based study using the National claim registry. Retina. 2017;37:2326–33.
Lieberman RM, Gow JA, Grillone LR. Development and implementation of a vitreous hemorrhage grading scale. Retin Physician. 2006;3:S1–S8.
Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112:159–65.
Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247:137–42.
Chen SN, Lian IeB, Wei YJ. Epidemiology and clinical characteristics of rhegmatogenous retinal detachment in Taiwan. Br J Ophthalmol. 2016;100:1216–20.
Park SJ, Choi NK, Park KH, Woo SJ. Five year nationwide incidence of rhegmatogenous retinal detachment requiring surgery in Korea. PLoS One. 2013;8:e80174.
Williams KM, Dogramaci M, Williamson TH. Retrospective study of rhegmatogenous retinal detachments secondary to round retinal holes. Eur J Ophthalmol. 2012;22:635–40.
Boutin TS, Charteris DG, Chandra A, Campbell S, Hayward C, Campbell A, et al. Insights into the genetic basis of retinal detachment. Hum Mol Genet. 2020;29:689–702.
Mitry D, Singh J, Yorston D, Siddiqui MA, Wright A, Fleck BW, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118:1429–34.
Aylward GW, Cooling RJ, Leaver PK. Trauma-induced retinal detachment associated with giant retinal tears. Retina. 1993;13:136–41.
Mehdizadeh M, Afarid M, Hagigi MS. Risk factors for giant retinal tears. J Ophthalmic Vis Res. 2010;5:246–9.
Kwong TQ, Shunmugam M, Williamson TH. Characteristics of rhegmatogenous retinal detachments secondary to retinal dialyses. Can J Ophthalmol. 2014;49:196–9.
Kinyoun JL, W H Knobloch WH. Idiopathic retinal dialysis. Retina. 1984;4:9–14.
Abdolrahimzadeh S, Piraino DC, Scavella V, Abdolrahimzadeh B, Crucian F, Gharbiya M, et al. Spectral domain optical coherence tomography and B-scan ultrasonography in the evaluation of retinal tears in acute, incomplete posterior vitreous detachment. BMC Ophthalmol. 2016;16:60.
Shunmugam M, Shah AN, Hysi PG, Williamson TH. The pattern and distribution of retinal breaks in eyes with rhegmatogenous retinal detachment. Am J Ophthalmol. 2014;157:221–226.
Chen C, Huang S, Sun L, Li S, Huang L, Wang Z, et al. Analysis of etiologic factors in pediatric rhegmatogenous retinal detachment with genetic testing. Am J Ophthalmol. 2020;218:330–6.
Acknowledgements
BEAVRS and Euretina VR Retinal Detachment outcomes group: Atiq Babar, Hull and East Yorkshire Eye Hospital, Hull, UK; Kamaljit Singh Balaggan, Wolverhampton and Midland Counties Eye Infirmary, New Cross Hospital, Wolverhampton, UK; Anthony G Casswell, Brighton and Sussex University Hospitals NHS Trust, Brighton, UK; Aman Chandra, Mid & South Essex NHS Foundation Trust, Southend, UK, Anglia Ruskin University, Cambridge, UK; Stephen Charles, Manchester Royal Eye Hospital, Manchester, UK; Timothy Cochrane, Maidstone and Tunbridge Wells NHS Trust, Tunbridge Wells, UK; Niels Crama, Radboudumc, Nijmegen, The Netherlands; Sandro Di Simplicio Cherubini, Newcastle Eye Centre, Royal Victoria Infirmary, Newcastle upon Tyne, UK; Abdallah A Ellabban, Hull University Teaching Hospitals, Hull, UK; John Ellis, Ninewells Hospital, Dundee, UK; Peter van Etten, Retina Operation Center Utrecht, Utrecht, The Netherlands; Marta S. Figueroa, Ramon y Cajal University Hospital, Madrid, Spain, Alcala de Henares University, Madrid, Spain; Craig Goldsmith, James Paget University Hospitals NHS Trust, Great Yarmouth UK; Roxane J Hillier, Newcastle Eye Centre, Royal Victoria Infirmary, Newcastle upon Tyne, UK; Edward Hughes, University Hospitals Sussex, Brighton, UK; Tsveta Ivanova, Manchester Royal Eye Hospital, Manchester, UK; Assad Jalil, Manchester Royal Eye Hospital, Manchester, UK; Huw Jenkins, Hywel Dda University Health Board, Carmarthenshire, UK; Ashraf Khan, Princess Alexandra Eye Pavilion, Edinburgh, UK; D Alistair Laidlaw, Guy’s and St. Thomas’ NHS Foundation Trust, London, UK; Yannick Le Mer, Hopital Fondation A. de Rothschild, Paris, France; Angelina Meireles, Centro Hospitalar Universitário do Porto, Porto, Spain; Andrew HC Morris, Royal Bournemouth Hospital, Bournemouth, UK; Richard Newsom, University of Portsmouth, Portsmouth, UK; Vasileios T Papastavrou, Cumberland Infirmary, Carlisle, UK; Jonathan C Park, Musgrove Park Hospital, Taunton, UK; Yashin D Ramkissoon, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK; Diego Sanchez-Chicharro, Martin University Hospital, Martin, Slovakia; Richard Sheard, Derwent Eye Specialists, Hobart, Tasmania; Jonathan Smith, Sunderland Eye Infirmary, Sunderland, UK; Kurt Spiteri Cornish, Sheffield Teaching hospitals NHS Trust, Sheffield, UK; David HW Steel, Sunderland Eye Infirmary, Sunderland, UK; Vaughan Tanner, King Edward VII Hospital, Windsor, UK; Deepak Vayalambrone, East Suffolk and North Essex NHS Foundation Trust, Essex, UK; Tom H Williamson, Guy’s and St. Thomas’ NHS Foundation Trust, London, UK; Stephen Winder, Royal Hallamshire Hospital, Sheffield; David Yorston, Gartnavel Hospital, Glasgow, UK. No funding has been received for this work.
Author information
Authors and Affiliations
Consortia
Contributions
DHWS contributed to the design of the study, interpretation of the data, the correction of the draft, and the final revision. PA analysed the data. DY, THW, DAL and MF contributed to the interpretation of the data. MF, AS, MAZ wrote the first draft and created the tables and the figures. All authors provided feedback on the draft, revised the final version, read and approved the final manuscript. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferrara, M., Al-Zubaidy, M., Song, A. et al. The effect of age on phenotype of primary rhegmatogenous retinal detachment. Eye 37, 1114–1122 (2023). https://doi.org/10.1038/s41433-022-02061-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02061-y
This article is cited by
-
The rising tide of rhegmatogenous retinal detachment in Germany: a nationwide analysis of the incidence, from 2005 to 2021
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
The effect of sex and laterality on the phenotype of primary rhegmatogenous retinal detachment
Eye (2023)