Abstract
Objectives
To establish a potential relationship between diabetic retinopathy (DR) and different stages of cognitive impairment
Methods
Literature searches were conducted on PubMed and EMBASE, with keywords “diabetic retinopathy” and “cognitive impairment”. Inclusion criteria were original human studies, and English language. Quality of studies was assessed by the Newcastle-Ottawa Quality Assessment (NOSGEN). The register number of this study on the International Prospective Register of Systematic Reviews (PROSPERO) is CRD42021236747. The main outcome measures were odds ratios (OR) and risk ratios (RR) for cross-sectional and longitudinal studies, respectively. Meta-regression was performed to evaluate the effects of potential moderator variables, including, age, onset age of diabetes mellitus (DM), duration of DM, and HbA1c.
Results
Twenty-five studies (17 cross-sectional and 8 longitudinal studies) with a total of 1,963,914 subjects, were included. Among the cross-sectional studies, the pooled ORs of any cognitive impairment, early stage of cognitive impairment and dementia in subjects with DR (95% confidence interval) were 1.48 (1.08–2.02), 1.59 (1.01–2.51), and 1.13 (0.86–1.50), respectively. Among the longitudinal studies, the pooled RRs of any cognitive impairment, early stage of cognitive impairment, and dementia in subjects with DR (95% confidence interval) were 1.35 (1.12–1.65), 1.50 (1.06–2.12), and 1.31 (1.03–1.66), respectively. Meta-regression showed age, onset age of DM, duration of DM, and glycated hemoglobin (HbA1c) were not statistically associated with the outcomes.
Conclusions
The presence of DR in DM patients indicates both higher odds of prevalent cognitive impairment and escalated risks of developing cognitive impairment in the future.
摘要
目的
建立糖尿病视网膜病变(DR)与不同阶段认知障碍之间的潜在关联。
方法
PubMde 和 EMBASE数据库中使用“糖尿病视网膜病变”和“认知障碍”两个关键词进行文献检索。纳入标准为临床研究和英文文献, 我们通过Newcastle-Ottawa质量评估(NOSGEN)方法评估研究质量。本研究在国际前瞻性系统性综述登记研究(PROSPERO)中的登记研究编号为CRD42021236747。比值比 (OR) 和风险比 (RR) 分别是横断面研究和纵向研究的主要结局指标。荟萃-回归分析用以评估潜在调节变量带来的影响, 包括年龄、糖尿病(DM)发病年龄、DM病程和HbA1c。
结果
共纳入25项研究 (17项横断面研究和8项纵向研究), 一共1, 963, 914例受试对象。在横断面研究中, DR受试者中任何认知障碍、早期认知障碍及痴呆的合并OR (95%置信区间) 分别为1.48(1.08–2.02)、1.59(1.01–2.51)和1.13(0.86–1.50)。在纵向研究中, DR受试者中任何认知障碍、早期认知障碍及痴呆的合并RR (95%置信区间) 分别为 1.35 (1.12–1.65), 1.50 (1.06–2.12) and 1.31 (1.03–1.66)。荟萃-回归分析显示, 年龄, DM发病年龄, DM病程和糖化血红蛋白 (HbA1c) 与认知障碍结局无统计学相关性。
结论
并发DR的DM患者发生认知障碍的几率高, 且将来发生认知障碍的风险也高。
Similar content being viewed by others
Introduction
With an ageing population, the world sees an emerging public health challenge of both diabetes mellitus (DM) and dementia [1,2,3]. In the recent report of the Lancet Commission, DM was attributed as one of the twelve modifiable risks factors for dementia in late life, associated with a population-attributable fraction of 1.1% [2, 4, 5]. In general, DM patients are in 1.53 times risk of incident dementia, compared to general population [5, 6]. However, a study has shown that DM patients with disease onset of 10 years earlier are in 2.12 times risk of incident dementia, compared with general population in the age group of 70 [7]. In view of a large number of diabetics, additional risk factors are warranted to further stratify diabetics with a higher risk of developing dementia.
While the precise mechanisms underlying the association between DM and dementia remain unclear, growing evidence has highlighted the role of microvascular dysfunction and the disruption of normal neurovascular coupling [8,9,10]. Therefore, one current area of interest is the association between the presence of diabetic retinopathy (DR) and dementia. Given that the retina is an extension of the central nervous system [11,12,13,14,15], the presence of DR might implicate dysfunction of the neurovascular unit in the central nervous system and hence indicates an escalated risk of dementia [16, 17].
However, the findings from the previous studies are not entirely consistent. For example, DR has been reported as an independent predictor for cognitive impairment in a longitudinal study [18], which was further confirmed by a 32-year follow-up in the diabetes control and clinical trials (DCCT) and epidemiology of diabetes interventions and complications (EDIC) study [19]. In contrast, Crosby-Nwaobi et al. discovered an inverse relationship of the severity of DR to cognitive impairment [20].
Despite two previous meta-analyses attempted to resolve the relationship between DR and dementia [21, 22], the following issues have not been addressed. First, the association between DR and different stages of cognitive impairment remains unclear. Whilst dementia is the end-stage of cognitive impairment with impairment of the activity of daily living, mild cognitive impairment (MCI) [23] and cognitive impairment no dementia (CIND) [24] describe the pre-dementia phase with a three to fivefold increase in risk of progressing into dementia compared to the general population [25]. It is important to determine whether DR is associated with early cognitive impairment as therapeutic intervention applied earlier in the course of dementia would be more likely to achieve disease modification [26, 27]. Second, the manifestation of microvascular complications in DM depends heavily on a number of variables, such as age of DM onset, DM duration and glycated hemoglobin (HbA1c). Adjustment of these variables is essential to confirm whether DR and cognitive impairment is associated independently.
Therefore, we conducted this systematic review and meta-analysis to comprehensively evaluate the association between DR and different stages of cognitive impairment among patients with DM.
Subjects and methods
Eligibility criteria
A systematic review and meta-analysis to evaluate the relationship between DR and cognitive impairment was performed according to the Meta-analysis of Observation Studies in Epidemiology guideline [28]. The register number of this study on the International Prospective Register of Systematic Reviews (PROSPERO) is CRD42021236747. To be included in the current meta-analysis, a study had to meet the following inclusion criteria: (1) an original human study with a case–control, cross-sectional or prospective design; (2) a study including: (a) DM subjects with DR with or without cognitive impairment, MCI, CIND, dementia, and (b) DM subjects without DR with or without these neurodegenerative conditions; (3) abovementioned ophthalmological and cognitive conditions confirmed by specialists, following established diagnostic systems (e.g., The International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis codes, the Third Revised Edition and Fourth Edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-IIIR, DSM-IV)). (4) English-written full-text manuscripts published in peer-reviewed journals before 9 July 2021. We excluded letters to the editor, commentaries, notes, case reports, case series, authors’ replies, and conference abstracts.
Search methods
Three independent reviewers (Chan RNF, Chan RNC and Cheng ETW) conducted a literature search in PubMed and Excerpta Medica Database (EMBASE). A hierarchical search strategy with the use of medical subject headings was adopted for the PubMed query. One of the following items: “Diabetic Retinopathy [MeSH Terms]”, “Diabetic Retinopathy”, “Diabetes Mellitus [MeSH Terms] AND Retinopathy[MeSH Terms]” or “Diabetes Mellitus AND Retinopathy” was first searched, followed by refinement with one of the below-listed keywords: “Alzheimer Disease”[Mesh], “Alzheimer Disease”, “Dementia [MeSH Terms]”, “Dementia”, “Neurobehavioral Manifestations [MeSH Terms]”, “Neurobehavioral Manifestations”, “Cognitive Dysfunction [MeSH Terms]”, “Cognitive Dysfunction”, “Cognitive Impairment”, and “Cognitive Decline”. For EMBASE query, a search enquiry of “Diabetic Retinopathy or “Diabetes mellitus AND Retinopathy” was created, followed by refinement of all the following items linked with “OR”: “Alzheimer Disease”, “Dementia”, “Mild Cognitive Impairment (MCI)”, “Cognitive Defect”, “Cognitive Impairment. mp.”, “Cognitive Dysfunction. mp.” and “Cognitive Decline. mp.” The detailed search strategy is listed in Supplementary Information, Tables 1, 2. To avoid missing relevant articles, we also manually searched the bibliographies of eligible studies.
Study selection
A two-phase selection process was adopted to identify eligible studies. First, the three independent reviewers screened the titles and abstracts of all articles identified in the literature search and excluded all ineligible studies which were irrelevant to the relationship between DR and cognitive impairment. Then, the reviewers assessed the full texts of the remaining studies, during which studies were excluded if inclusion criteria were not fully fulfilled, or any exclusion criteria were met. In the whole study selection process, disagreements between the three reviewers were resolved by discussions with a senior reviewer (CYC).
Data collection and risk of bias assessment
Data from eligible studies were extracted into a customized database, including first author’s name and title of study, year of publication, subject category (controls, MCI or CIND and dementia), sample size, gender, mean age, mean HbA1c and the duration of DM. All data were acquired from the published articles without additional information from the authors of their respective studies. If appropriate, unavailable data were calculated by the built-in program of RevMan (version 5.4; Cochrane Collaboration, London, United Kingdom) whenever necessary. Newcastle-Ottawa Quality Assessment Scale (NOSGEN) was adopted to evaluate the study quality of the included literature (Supplementary Information, Note 1) [29]. NOSGEN was comprised of three domains: Patient Selection, Comparability and Outcomes, which was used in the scale for cohort and case–control studies. However, some of the cross-sectional studies were not conducted in a case–control design, so a modified version of NOSGEN developed by Modesti et al. was used in this review (Supplementary Information, Note 2) [30].
Data synthesis and analysis
We performed statistical analyses with RevMan (Version 5.4). Odds ratio (OR) was used to analyse the cross-sectional relationship of DR and any cognitive impairment, whereas risk ratio (RR) was used in the longitudinal studies. All meta-analyses were performed under random-effect models.
We first analysed the association between the presence of DR and any cognitive impairment. Then, we separately analysed the associations of DR with early cognitive impairment (i.e., MCI or CIND) and dementia. Additionally, we also assessed whether the severity of DR affects the association between DR and cognitive impairment.
To investigate the effect of moderator variables on the effect sizes, we further constructed a random effects meta-regression adjusting for the mean age of onset of DM, mean duration of DM and mean HbA1c, using R (Version 4.0.5).
Results
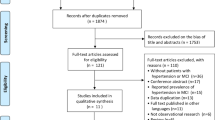
Figure 1 summarizes the selection process for all studies included in this meta-analysis. Amongst 2006 articles identified in the initial literature search (1117 from PubMed and 889 from EMBASE), 1209 duplicates were removed. The remaining 797 articles were screened by reviewing their titles and abstracts, where 650 studies of irrelevant topics were excluded. We then reviewed the full text of the 147 articles left, of which 122 were excluded on account of meeting the exclusion criteria or same study population in different publications. In the latter case, if the number of subjects included was different, the one with more subjects included would be selected; if the number of subjects included was the same, the latest one would be selected. In addition, Crosby-Nwaobi et al. published three articles with different subjects from the same cohort in 2014, 2015, and 2016 [31,32,33]. To avoid double-counting, the one with the largest number of included patients was included in this paper [20]. Despite a recent study by Jacobson et al. identified DR as an independent risk factor in a 32-year follow-up of the DCCT and EDIC study, the study was not included in our analysis due to the unavailability of the number of DR patients with or without cognitive impairment [19].
25 studies with a total of 1,963,914 subjects (1,760,013 diabetic subjects without DR and 203,901 subjects with mild to severe DR) were finally included in the current meta-analysis. 17 were in cross-sectional design and 8 were in longitudinal design, of which 1 provided both cross-sectional and longitudinal data. The mean age of included studies ranged from 49.1 to 80.3 and the average age of all the included subjects was 59.9 (±1.50). The quality assessment of included studies indicated a generally high quality of the included studies as shown in Supplementary Information, Table 3. Details of the included studies are summarized in Supplementary Information, Table 4.
Table 1 summarizes the meta-analysis of cross-sectional associations between DR and cognitive impairment, while Table 2 summarizes the meta-analysis of longitudinal associations between DR and cognitive impairment.
Association between DR and any cognitive impairment
Eighteen cross-sectional studies assessed the association between DR and any cognitive impairment. Among those with DM, subjects with DR were associated with 1.48 times in the odds with any cognitive impairment compared with those without DR (OR, 1.48, 95% CI, 1.08–2.02), despite a significant heterogeneity (I2 = 73%, P < 0.001) (Fig. 2). Eight longitudinal studies assessed the association between DR and any cognitive impairment. The risk of any cognitive impairment amongst DR subjects was 1.35 times that amongst non-DR subjects (RR, 1.35, 95% CI, 1.12–1.65). A significant heterogeneity was observed (I2 = 92%, P < 0.001) (Fig. 3). We did not observe any significant effect of moderator variables, including mean age, mean onset age of DM, mean duration of DM, and mean HbA1c on the association between DR and the risk of developing any cognitive impairment (Supplementary Information, Table 5).
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
Association between DR and early stage of cognitive impairment
Twelve cross-sectional studies assessed the association between DR and early stage of cognitive impairment (i.e., MCI or CIND). Subjects with DR were associated with 1.59 times in the odds with early stage of cognitive impairment compared with diabetic subjects without DR (OR, 1.59, 95% CI, 1.01–2.51), despite a significant heterogeneity (I2 = 80%, P < 0.001) (Fig. 4). Three longitudinal studies assessed the association between DR and early stage of cognitive impairment. The risk of any cognitive impairment amongst DR subjects was 1.50 times that amongst non-DR subjects (RR, 1.50, 95% CI, 1.06–2.12). No significant heterogeneity was observed (I2 = 0%, P = 0.63) (Fig. 5).
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
Association between DR and dementia
Six cross-sectional studies assessed the association between DR and dementia. Subjects with DR were not significantly associated with dementia compared with diabetic subjects without DR (OR, 1.13, 95% CI, 0.86–1.50) (Fig. 6). Five longitudinal studies assessed the association between DR and dementia. The risk of any cognitive impairment amongst DR subjects was 1.31 times that amongst non-DR subjects (RR, 1.31, 95% CI, 1.03–1.66). Significant heterogeneity was observed (I2 = 95%, P < 0.001) (Fig. 7).
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
Association between severity of DR and any cognitive impairment
Four cross-sectional studies assessed the association between PDR and any cognitive impairment, which was found to be statistically insignificant in our meta-analysis (OR, 0.87, 95% CI, 0.49–1.52) (Fig. 8).
The meta-analysis was performed by a random effects model. The size of the box is related to the weight that given to that particular study. The horizontal lines correspond to the 95% confidential intervals. The peaks of diamonds represent the overall effect estimate and the edges of that represent the 95% confidential intervals.
Discussion
This systematic review and meta-analysis, including 18 cross-sectional studies with 5924 subjects and 8 longitudinal studies with 1,957,973 subjects, provided robust evidence that the presence of any DR is associated with both the prevalence and incidence of any cognitive impairment.
To further dissect the relationship between DR and cognitive impairment, we analysed the relationship of any DR with early cognitive impairment and dementia respectively. We illustrated that the presence of any DR was associated with the prevalence and incidence of early cognitive impairment. However, the presence of DR only increased the risk of developing incident dementia, but it was not associated with the prevalence of dementia. The associations between DR and cognitive impairment might be explained by considering DM as a slowly progressive metabolic disorder that affects the neurovascular unit of both the retina and the brain in parallel [16, 34]. In the CNS including the retina, neurons, glia and the highly specialized vasculature work in concert as a neurovascular unit to maintain the normal homeostasis whilst dynamically regulating blood flow in response to metabolic demands of the neurons. The dysfunction of “neurovascular coupling” is not only a key feature of early stage of DR [35,36,37,38,39,40], but also found in dementia [16, 41,42,43,44], as suggested by the presence of DM-related vascular changes, such as narrowing of arteries, blood-brain barrier leakiness and thickening of basement membrane with pericytes detachment [45, 46]. The microvascular dysfunction also contributes to dementia by disrupting amyloid-beta clearance across the BBB and leading to overexpression of amyloid-beta precursor protein, both of which promote amyloid-beta accumulation [41, 47, 48].
Our findings highlighted DR as a risk factor for cognitive impairment in DM population. As cognitive impairment decreases the short-term memory and daily problem-solving ability of patients [49], it affects DM self-management, such as self-monitoring of blood glucose level or insulin self-injection [50]. This leads to poor glycaemic controls, which in turns worsen the development of cognitive impairment, forming a vicious cycle between DM and cognitive impairment [51]. As a result, it is important to stratify patients with higher risk of cognitive impairment so that a more frequent follow-up and more aggressive glycaemic control can be provided to reduce the risk of progressing into more severe form [52]. Apart from the association between DR and cognitive impairment found in our studies, screening for DR from retinal photographs using artificial intelligence (AI) is an emerging trend [53,54,55]. This raises the potential practicality of utilizing AI to identify DR and assess risk for cognitive impairment subsequently. Nevertheless, further research is essentially needed.
Besides, it is noteworthy to mention that age of DM onset, DM duration, and HbA1c did not influence on the association between DR and the risk of developing any cognitive impairment. Patients presenting with retinopathy, regardless of age and disease duration, might benefit from subsequent assessment of cognitive function.
This meta-analytic review summarized the inconsistent findings, and established the association between DR and cognitive impairment, with a large number of studies included. However, the current study included several limitations. First, no diagnostic biomarkers or neuroimaging were currently available for assessment of cognitive impairment in these studies, which might potentially lead to misclassification bias. Second, different studies used different cognitive assessment tools to define the cognitive capacity of subjects which may also confound the findings of our study. Third, there were considerable heterogeneity between the studies, as the development of dementia is usually multi-factorial. We tried to explore the reasons behind the high heterogeneity using meta-regression. However, given the nature of the analysis, our meta-analysis with <10 studies included cannot provide the reliable results. Forth, we were not able to perform sub-group analysis on type 1 and type 2 DM, because most of the included studies did not provide separate data on the type of DM patients included.
In conclusion, the presence of DR is significantly associated with the prevalence and incidence of any cognitive impairment, independent of the age of DM onset, DM duration, and HbA1c level. The relationship of DR with the prevalent and incident early-stage cognitive impairment is confirmed. This suggests that DR may serve as a risk factor in DM for identifying patients at higher risk for cognitive impairment.
References
Wong TY, Sabanayagam C. The war on diabetic retinopathy: where are we now? Asia Pac J Ophthalmol. 2019;8:448–56. https://doi.org/10.1097/APO.0000000000000267.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
World Health Organization. Dementia [electronic resource]: a public health priority. Geneva: World Health Organization; 2012.
Livingston GO, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, et al. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract. 2017;124:41–7. https://doi.org/10.1016/j.diabres.2016.10.024.
Biessels GJ, Nobili F, Teunissen CE, Simó R, Scheltens P. Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol. 2020;19:699–710. https://doi.org/10.1016/S1474-4422(20)30139-3.
Amidei CB, Fayosse A, Dumurgier J, Machado-Fragua MD, Tabak AG, van Sloten T, et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA. 2021;325:1640–9. https://doi.org/10.1001/jama.2021.4001.
Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–9.
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74.
van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8:325–36. https://doi.org/10.1016/S2213-8587(19)30405-X.
London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. https://doi.org/10.1038/nrneurol.2012.227.
Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, et al. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci. 2016;10:20. https://doi.org/10.3389/fncel.2016.00020.
Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51. https://doi.org/10.1016/j.preteyeres.2015.06.003.
Cheung CY, Chan VTT, Mok VC, Chen C, Wong TY. Potential retinal biomarkers for dementia: what is new? Curr Opin Neurol. 2019;32:82–91. https://doi.org/10.1097/WCO.0000000000000645.
Cheung CY-L, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res. 2017;57:89–107. https://doi.org/10.1016/j.preteyeres.2017.01.001.
van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, et al. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. http://europepmc.org/abstract/MED/2944676910.1038/nrdp.2018.3.
Hendrikx D, Smits A, Lavanga M, De Wel O, Thewissen L, Jansen K, et al. Measurement of Neurovascular Coupling in Neonates. Front Physiol. 2019;10. https://doi.org/10.3389/fphys.2019.00065.
Bruce DG, Davis WA, Starkstein SE, Davis TM. Mid-life predictors of cognitive impairment and dementia in type 2 diabetes mellitus: the Fremantle Diabetes Study. J Alzheimers Dis. 2014;42:S63–70. https://doi.org/10.3233/JAD-132654.
Jacobson AM, Ryan CM, Braffett BH, Gubitosi-Klug RA, Lorenzi GM, Luchsinger JA, et al. Cognitive performance declines in older adults with type 1 diabetes: results from 32 years of follow-up in the DCCT and EDIC Study. Lancet Diabetes Endocrinol. 2021. https://doi.org/10.1016/S2213-8587(21)00086-3.
Crosby-Nwaobi RR, Sivaprasad S, Amiel S, Forbes A. The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care. 2013;36:3177–86. https://doi.org/10.2337/dc12-2141.
Pedersen HE, Sandvik CH, Subhi Y, Grauslund J, Pedersen FN. Relationship between diabetic retinopathy and systemic neurodegenerative diseases: a systematic review and meta-analysis. Ophthalmol Retina. 2021. https://doi.org/10.1016/j.oret.2021.07.002.
Cheng D, Zhao X, Yang S, Wang G, Ning G. Association between diabetic retinopathy and cognitive impairment: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13. https://doi.org/10.3389/fnagi.2021.692911.
Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2017;174:329–40. https://doi.org/10.1176/appi.ajp.2016.16030360.
Jacova C, Peters KR, Beattie BL, Wong E, Riddehough A, Foti D, et al. Cognitive impairment no dementia—neuropsychological and neuroimaging characterization of an amnestic subgroup. Dement Geriatr Cogn Disord. 2008;25:238–47. https://doi.org/10.1159/000115848.
Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. 2013;29:873–93. https://doi.org/10.1016/j.cger.2013.07.009.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. https://doi.org/10.1016/j.jalz.2011.03.003.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 3 Feb 2020.
Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0147601. https://doi.org/10.1371/journal.pone.0147601.
Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab Brain Dis. 2016;31:257–66. https://doi.org/10.1007/s11011-015-9739-0.
Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J. C-reactive protein, advanced glycation end products, and their receptor in type 2 diabetic, elderly patients with mild cognitive impairment. Front Aging Neurosci. 2015;7:209. https://doi.org/10.3389/fnagi.2015.00209.
Gorska-Ciebiada M, Saryusz-Wolska M, Ciebiada M, Loba J. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res. 2014;2014:179648. https://doi.org/10.1155/2014/179648.
Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–90. https://doi.org/10.1038/s41581-020-0278-5.
Moran EP, Wang Z, Chen J, Sapieha P, Smith LEH, Ma J-X. Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications. Am J Physiol Heart Circ Physiol. 2016;311:H738–49. https://doi.org/10.1152/ajpheart.00005.2016.
Gardner T, Davila J. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255. https://doi.org/10.1007/s00417-016-3548-y.
Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2:e93751. https://doi.org/10.1172/jci.insight.93751.
Nakahara T, Mori A, Kurauchi Y, Sakamoto K, Ishii K. Neurovascular interactions in the retina: physiological and pathological roles. J Pharmacol Sci. 2013;123:79–84. https://doi.org/10.1254/jphs.13R03CP.
Nian S, Lo ACY, Mi Y, Ren K, Yang D. Neurovascular unit in diabetic retinopathy: pathophysiological roles and potential therapeutical targets. Eye Vis. 2021;8:15. https://doi.org/10.1186/s40662-021-00239-1.
Tang Z, Chan MY, Leung WY, Wong HY, Ng CM, Chan VTT, et al. Assessment of retinal neurodegeneration with spectral-domain optical coherence tomography: a systematic review and meta-analysis. Eye. 2021;35:1317–25. https://doi.org/10.1038/s41433-020-1020-z.
Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. https://doi.org/10.1038/nrn3114.
Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15:934–43. https://doi.org/10.1016/S1474-4422(16)30029-1.
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Weiner MW, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. https://doi.org/10.1038/ncomms11934.
Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta. 2016;1862:887–900. https://doi.org/10.1016/j.bbadis.2015.12.016.
Alzheimer’s A. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. https://doi.org/10.1016/j.jalz.2016.03.001.
Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: JACC focus seminar. J Am Coll Cardiol. 2020;75:942–51. https://doi.org/10.1016/j.jacc.2019.10.062.
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–78. https://doi.org/10.1016/j.cell.2015.10.067.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–34. https://doi.org/10.1038/nrn.2017.48.
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. https://doi.org/10.1016/j.jalz.2016.02.002.
Beck J, Greenwood DA, Blanton L, Bollinger ST, Butcher MK, Condon JE, et al. National standards for diabetes self-management education and support. Diabetes Care. 2017;40:1409–19. https://doi.org/10.2337/dci17-0025.
Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J Behav Med. 2012;35:190–9. https://doi.org/10.1007/s10865-011-9344-6.
Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. Glucose levels and risk of dementia. New Engl J Med. 2013;369:540–8. https://doi.org/10.1056/NEJMoa1215740.
Ruamviboonsuk P, Cheung CY, Zhang X, Raman R, Park SJ, Ting DSW. Artificial intelligence in ophthalmology: evolutions in asia. Asia Pac J Ophthalmol. 2020;9:78–84.
Bellemo V, Lim G, Rim TH, Tan GSW, Cheung CY, Sadda S, et al. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr Diabetes Rep. 2019;19:72. https://doi.org/10.1007/s11892-019-1189-3
Cheung CY, Tang F, Ting DSW, Tan GSW, Wong TY. Artificial intelligence in diabetic eye disease screening. Asia Pac J Ophthalmol. 2019;8:158–64.
Funding
Health and Medical Research Fund, Hong Kong (Grant Number: 04153506). The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
RNFC was responsible for registering the protocol, conducting literature search, screening potentially eligible studies, extracting and analysing data, interpreting results, and writing the paper. ZT and VTTC contributed to data analysis, results interpretation, and writing the paper. RNCC, ETWC, and NCYN were responsible for designing the review protocol, writing the protocol, screening potentially eligible studies, and extracting and analysing data. CYC was responsible for designing the study, provided instructions on the paper, and supervised the entire process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chan, R.N.F., Tang, Z., Chan, V.T.T. et al. The cross-sectional and longitudinal relationship of diabetic retinopathy to cognitive impairment: a systematic review and meta-analysis. Eye 37, 220–227 (2023). https://doi.org/10.1038/s41433-022-02033-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02033-2
This article is cited by
-
Cognitive dysfunction in diabetes-related foot complications: A cohort study
Journal of Diabetes & Metabolic Disorders (2024)