Abstract
Purpose
To evaluate the changes of corneal biomechanics in granular, lattice and macular corneal dystrophy (GCD, LCD and MCD), and to assess the agreement of intraocular pressure (IOP) between Corvis ST tonometer (CST) and Goldmann applanation tonometer (GAT) and the agreement of central corneal thickness (CCT) between CST and ultrasound pachymeter (USP) in patients with corneal dystrophy.
Methods
Fifty-nine eyes with corneal dystrophy (26 eyes with GCD, 18 eyes with LCD and 15 eyes with MCD) and 48 eyes from healthy subjects were included in this study. All subjects received ocular examination and anterior segment photography under slit-lamp microscope. Corneal biomechanical parameters were obtained using CST. IOP and CCT were obtained using GAT and USP, respectively. Mixed-effects models were fitted for group comparisons and Bland-Altman analyses were applied for assessing the agreement of IOP or CCT between devices.
Results
GCD, LCD and MCD showed higher First Applanation Deformation Amplitude (A1DA) and Corvis Biomechanical Index (CBI), and a lower Stiffness Parameter at First Applanation (SPA1), compared to controls. After CCT adjustment, MCD group showed a higher A1DA compared to GCD or LCD. The IOP measured by CST demonstrated an overestimated bias to the one obtained by GAT in all groups. The CCT measured by CST and USP showed good agreement in healthy eyes but not in those with corneal dystrophy.
Conclusion
Corneal biomechanical alterations were observed in GCD, LCD and MCD. IOP and CCT measured by CST should be interpreted carefully in eyes with corneal dystrophy.
Similar content being viewed by others
Introduction
The cornea exhibits biomechanical characteristics including elasticity and viscosity [1]. Normal biomechanical properties of the cornea are important to maintain the structure and visual functions of the eye. Highly organised stromal collagen fibrils contribute to the corneal shape and stiffness [2]. Any damage or change of the stromal structure can induce alterations of the corneal biomechanics. Changes of corneal biomechanics have been demonstrated in many ocular and systemic conditions, including keratoconus [1], refractive surgery [3], corneal cross-linking [4] and diabetes mellitus [5].
Corneal dystrophy is a group of inherited disorders that are characterised by abnormal accumulations of deposits at different layers of the cornea [6]. Musch et al. reviewed records of 8 million enrolees in a national managed-care network in the United States and found the overall prevalence rate of corneal dystrophy was 897 per million covered lives [7]. Considering the insoluble deposits and the disorganised stromal structure in corneal dystrophy, it is reasonable to hypothesise that the corneal biomechanics are changed.
However, it was difficult to evaluate the corneal biomechanics in vivo, especially to obtain accurate quantified properties. Until 2005, the ocular response analyser (ORA) was introduced to assess corneal biomechanics, providing two corneal deformation response parameters, corneal hysteresis and corneal resistance factor [8]. The Corvis ST (CST; Oculus, Wetzlar, Germany), introduced in recent years, is a noncontact tonometer capable of quantifying the deformation of the cornea [9, 10]. It differs from the ORA by using high-speed Schempflug imaging to report the corneal biomechanical characteristics. Some studies found that CST parameters were poorly correlated with those from ORA [10] and CST may be more useful in revealing true biomechanical differences [11].
There is evidence that the corneal hysteresis and corneal resistance factor are reduced in Fuchs’ endothelial dystrophy [12]. A recent case report described the association between the protein deposits in lattice corneal dystrophy and the increased corneal biomechanical stiffness [13]. To our best knowledge, however, no previous study investigated the corneal biomechanics in other types of corneal dystrophy. Granular, lattice and macular corneal dystrophy (GCD, LCD and MCD) are three of the most common types of corneal dystrophy, presenting distinct abnormal deposits in the corneal stroma. Understanding the corneal biomechanical changes in corneal dystrophy is beneficial to further elucidate the relationship between corneal biomechanics and stromal structure. Therefore, in this study, we utilised the CST to explore the corneal biomechanical changes in GCD, LCD and MCD and the potential difference between them. In addition, the agreement between CST and Goldmann applanation tonometer or ultrasound pachymeter was compared for measuring intraocular pressure (IOP) or corneal central thickness (CCT).
Materials and methods
Patients
A total of 35 adult patients (age ≥ 18 years) with a clinical diagnosis of granular corneal dystrophy (GCD), macular corneal dystrophy (MCD) or lattice corneal dystrophy (LCD) attending the Ocular Surface Subspecialty Clinic of the Eye, Ear, Nose, and Throat Hospital of Fudan University (Shanghai, China) were recruited. All patients presented typical clinical corneal appearances under slit-lamp microscope. Patients with any other ocular diseases or systemic diseases that may affect the eye including diabetes mellitus, thyroid disease or autoimmune diseases, were excluded. Representative photographs of the corneal appearance are shown in Supplementary Material Fig. S1. Twenty-four adult subjects (age ≥ 18 years) were recruited as a control group. Inclusion criteria for the healthy control subjects were (1) absence of current ocular diseases or systemic diseases that may affect the eye; (2) absence of a history of ocular surgery or trauma, or a history of contact lens wearing in the previous two months; (3) a best corrected visual acuity (BCVA) ≥ 20/20 with a spherical equivalent < −3.0 dioptres and cylinder power <1.0 dioptres; (4) a normal IOP (10–21 mmHg). Informed consent was obtained from all subjects. Data collection was approved by the Institutional Review Board Committee in accordance with the Declaration of Helsinki.
Ocular measurements
All the subjects underwent the non-invasive examinations prior to the relatively invasive procedures. Axial length and CCT were measured using an ultrasound pachymeter (USP; Tomey, Nagoya, Japan). IOP was measured using a Goldmann applanation tonometer (GAT) by an experienced ophthalmic technician.
Biomechanical parameters
Corneal biomechanical parameters were evaluated by CST. With the constant air puff provided by CST, the cornea moves inwards until it reaches a concavity phase and then moves back to original phase (Fig. 1a). The main parameters provided by CST include First/Second Applanation Time (A1T/A2T), First/Second Applanation Velocity (A1V/A2V), First/Second Applanation Deformation Amplitude (A1DA/A2DA), highest concavity Time (HCT) and Highest Concavity Deformation Amplitude (HCDA). In addition, a novel index, stiffness parameter at first applanation (SPA1), was developed for assessing the strength of the cornea [14]. Corvis biomechanical index (CBI) was also introduced to describe the corneal stiffness by combining different dynamic biomechanical parameters [15]. Those corneal biomechanical parameters are summarised in Supplementary Material Table S1. Biomechanics-corrected IOP (CST-IOP) and CCT (CST-CCT) measurements were also obtained by CST. All subjects underwent three successive measurements and the averaged values were recorded.
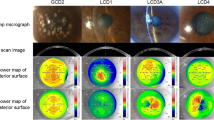
a Transverse plane of healthy cornea and schematic symbols show the corneal biomechanical parameters during four applanation stages, including initial place, first applanation, highest concavity and second applanation. A1DA, First Applanation Deformation Amplitude; HCDA, Highest Concavity Deformation Amplitude; A2DA, Second Applanation Deformation Amplitude; PD, Peak distance. b Transverse plane of cornea at the stage of first application in control, GCD, LCD and MCD.
Statistical analyses
Eyes with MCD, LCD or GCD were all included in the analysis. Both eyes of the healthy subjects were included as a control group. The data analysis was performed by fitting a linear mixed-effects model using restricted maximum likelihood (REML) and Kenward-Roger tests for fixed effects. The subject was included as a random effect to account for correlation between two eyes of a particular subject. After fitting the model, post-hoc tests with Sidak correction were performed to examine the fixed effect of different types of corneal dystrophy. Comparisons with CCT adjustment were performed as well. Inter-method agreement for the measurements of IOP and CCT was assessed using Bland-Altman analysis. The mean bias and Limits of Agreement (LoA, bias ± 1.96 standard deviations [SD]) were calculated. Pairwise correlation was tested using Pearson’s correlation coefficient. All statistical analyses were conducted by Stata 14.2 (StataCorp LLC, College Station, TX). P < 0.05 was considered to be statistically significant in all analyses. All summary data are shown as mean ± SD.
Results
Twenty-six eyes with GCD (14 subjects), 18 eyes with LCD (11 subjects), 15 eyes with MCD (10 subjects), and 48 control eyes (24 subjects) were included in this study. No difference was observed in age or sex between those groups. Mixed-effects models were used in the analysis to exclude the potential correlation between the two eyes of a particular subject. Table 1 showed the detailed comparison results. No difference was shown in axial length and GAT-IOP between the groups. The USP-CCT in MCD was 430.13 ± 74.42 µm, significantly thinner than the GCD, LCD and controls (all P < 0.001). With respect to the corneal biomechanical parameters, GCD, LCD and MCD groups showed a lower SPA1 and higher A1DA and CBI compared to controls. Representative CST images of corneal dystrophies are shown in Fig. 1b. Moreover, MCD group showed higher A1V and HCDA compared to the controls (p = 0.041 and 0.023, respectively).
In order to eliminate the effect of CCT on the corneal biomechanics, we compared the biomechanical parameters between the groups again but with an adjustment of CCT (based on USP-CCT). The CCT-adjusted p values are shown in Table 2. After the adjustment of CCT, eyes with GCD and LCD showed similar results in biomechanical parameters compared to control eyes. Eyes with MCD showed higher A1DA and CBI compared to controls (both p < 0.001). Interestingly, the A1DA in eyes with MCD was higher than GCD and LCD (p = 0.025 and 0.043, respectively).
The strength of correlation and level of discrepancy were compared for IOP and CCT measurements between the CST and GAT or USP. For the IOP measurement, there were significantly positive correlations between GAT and CST in controls and LCD but not in GCD and MCD groups (Fig. 2a1–a4). Bland-Altman analysis demonstrated overestimated bias in the IOP provided by CST compared to GAT, in controls (Fig. 2b1; p < 0.001, 95% LoA: −3.29 to 5.83 mmHg), GCD (Fig. 2b2; p < 0.001, 95% LoA: −2.54 to 5.83 mmHg), LCD (Fig. 2b3; p = 0.048, 95% LoA: −3.54 to 5.97 mmHg) and MCD group (Fig. 2b4; p < 0.001, 95% LoA: −0.88 to 8.43 mmHg).
a1–a4 scatter plot graphs show the correlation of intraocular pressure measurements between Corvis ST and Goldmann applanation tonometer in controls (a1), granular (a2), lattice (a3) and macular (a4) corneal dystrophy. b1–b4 Bland-Altman plot graphs show the agreement of intraocular pressure measurements between Corvis ST and Goldmann applanation tonometer in controls (b1), granular (b2), lattice (b3) and macular (b4) corneal dystrophy. IOP intraocular pressure, CST Corvis ST, GAT Goldmann applanation tonometer, LoA limits of agreement.
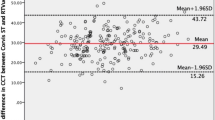
With respect to the CCT measurement, strong positive correlations were found between the CST and USP in control group (Fig. 3a1; r = 0.94, p < 0.001), GCD (Fig. 3a2; r = 0.87, p < 0.001) and MCD (Fig. 3a4; r = 0.87, p < 0.001), but not in LCD group (Fig. 3a3; r = 0.11, p = 0.653). Inter-method agreement analysis showed no significant difference between the CCTs measured by CST and USP in GCD and MCD groups (Fig. 3b2, b4; p = 0.286 and 0.918, respectively). Although in control group the CST showed an overestimated CCT compared to the USP, the bias was only 4.13 µm with a narrow 95% LoA of −12.60 to 20.85 µm (Fig. 3b1). In addition, the CST demonstrated a significant bias to underestimate the CCT in LCD group by 30.78 µm, compared to the USP (p = 0.025; 95% LoA: −135.00 to 73.48 µm) (Fig. 3b3).
a1–a4 Scatter plot graphs show the correlation of central corneal thickness measurements between Corvis ST and ultrasound pachymeter in controls (a1), granular (a2), lattice (a3) and macular (a4) corneal dystrophy. b1–b4 Bland-Altman plot graphs show the agreement of central corneal thickness measurements between Corvis ST and ultrasound pachymeter in controls (b1), granular (b2), lattice (b3) and macular (b4) corneal dystrophy. CCT central corneal thickness, CST Corvis ST, USP ultrasound pachymeter, LoA limits of agreement.
Discussion
The biomechanics of the cornea has attracted much attention in recent years. Many studies have demonstrated that CST exhibited a high repeatability in the measurements of CCT, IOP, A1V and HCDA [1, 16]. The measurements of corneal biomechanics provide a novel aspect to the diagnosis and progress evaluation of corneal diseases including corneal dystrophy. In this study, we investigated the corneal biomechanical in eyes with corneal dystrophies including GCD, LCD and MCD (Salient biomechanical indices are summarised in Supplementary Material Fig. S2). A significant increase of A1DA and alterations of two novel biomechanical indices, SPA1 and CBI, were observed in corneal dystrophies. Following the adjustment of CCT, the A1DA was significantly higher in eyes with MCD compared to LCD or GCD. In addition, the IOP provided by CST showed an overestimated bias compared to GAT in controls and corneal dystrophies. The CCT obtained by CST and USP may be interchangeable in normal eyes but not in those with MCD or LCD.
It is speculated that the first applanation reflects corneal elasticity while the second applanation is associated with corneal viscoelasticity [17, 18]. As an important parameter in biomechanics, A1DA represents sagittal deformation amplitude of apex at the first applanation. In this study, GCD, LCD and MCD groups all showed an increased A1DA, which indicates a decreased corneal elasticity. SPA1 and CBI are two novel indices that have been developed to describe the strength of the cornea by combining different dynamic biomechanical parameters [14, 15]. In patients with corneal dystrophies, we observed a decreased SPA1 and an increased CBI that both indicate a softer property of the cornea. Interestingly, similar alterations of SPA1 and CBI in corneal biomechanics were reported in patients with keratoconus [19]. Vinciguerra et al. demonstrated that CBI was highly sensitive and specific to distinguish ectatic corneas from controls to aid the diagnosis of keratoconus [15]. Although the International Committee for Classification of Corneal Dystrophies (IC3D) excludes corneal ectasias from the corneal dystrophy, some researchers believe that keratoconus should be included as a corneal dystrophy subtype based on the genetic pattern [6, 20]. In our study, we found that corneal dystrophies, especially MCD, showed a significant increase in CBI that is consistent with keratoconus. It has been reported that keratoconus and stromal dystrophy are the top two diagnoses that can develop Urrets-Zavalia syndrome (UZS, an uncommon postoperative complication with fixed dilated pupil) after surgical intervention [21]. In a total of 110 reported cases of UZS, 78.1% of the cases happened after the surgical intervention of keratoplasty [21]. Moreover, a retrospective study of >2000 patients with penetrating keratoplasty revealed that the incidence of UZS was higher in patients with MCD than those with keratoconus [22]. The pathogenesis of UZS in keratoconus was suggested as a result of the decreased corneal rigidity [21, 23], which is consistent with our finding in the corneal biomechanical properties in MCD. This evidence supported the assumption that MCD and keratoconus may share similar changes in corneal rigidity, which is associated with the pathogenesis of UZS. Corneal biomechanical alterations were also observed in subclinical keratoconus [24], while it needs further verification in different stages of MCD. The corneal biomechanics could be able to provide a new aspect to investigate their similarity and difference in further studies.
Interestingly, increased A1V and HCDA were observed in MCD but not after the adjustment of CCT, indicating that CCT did affect the biomechanical parameters including A1V and HCDA. Although some studies found that no CST parameters were influenced by CCT [25], Wang et al. reported that CCT was correlated with A1T, A1DA, A2V and HCDA in a healthy population [16]. Bhikoo et al. reported a decreased HCDA in a case of LCD [13]. However, this case showed a scarred cornea which may influence the biomechanics. One of the novel stiffness parameters, SPA1, also showed no difference between MCD and controls after CCT adjustment. This result is consistent with previous study that a strong correlation between SPA1 and CCT was demonstrated when this novel parameter was introduced [14]. Therefore, these parameters need to be interpreted carefully when there is a difference in CCT. Despite that, the A1DA and CBI showed similar results after the adjustment of CCT, indicating the two parameters might be more appropriate to evaluate the biomechanics in corneal dystrophy. Interestingly, after the CCT adjustment, the MCD group showed a significantly higher A1DA compared to GCD and LCD. Wang et al.’s study demonstrated a positive correlation between CCT and A1DA [16], providing strong evidence to the hypothesis that the difference of CCT might obscure corneal biomechanical changes such as A1DA in corneal dystrophy. The higher value of A1DA in MCD could have been more significant considering the thinner CCT in MCD.
The difference in corneal deposits and structure might explain the biomechanical difference observed between the three types of corneal dystrophy. GCD and LCD are both related with the mutation of TGFBI gene, and the corneal deposits develop initially in the superficial stroma while the Descemet membrane are normal. Moreover, the intervening stroma of GCD maintains transparent with normal collagen structure [6]. However, differ from GCD and LCD, the corneal deposits of MCD can involve deep stroma down to the Descemet membrane and endothelium with a progressive diffuse haze involves the entire corneal stroma [26]. The extensive microstructural alterations of stroma may contribute to the faster deformation velocity and increased deformation amplitude. Another explanation is that the thinner CCT of eyes with MCD contributes to the altered corneal biomechanics. Nevertheless, we have demonstrated that subjects with MCD presented an increased A1DA that is CCT-independent. Further studies are necessary to evaluate dynamic changes of corneal biomechanics in patients without distinct clinical corneal deposits and the relationship with the development of corneal dystrophy.
The biomechanics-corrected IOP provided by CST showed an overestimated tendency in patients with corneal dystrophy. Consistent with our results, studies have reported that CST provided an overestimated IOP in healthy and glaucoma patients [27, 28]. However, there was conflicting evidence that CST had a tendency to underestimate IOP compared to GAT [18]. In most studies, it is interchangeable when the IOP provided by a tonometer with a LoA within ±3 mmHg compared with GAT [18, 29, 30]. In our report, ~70% of eyes in GCD, LCD and healthy groups showed a ΔIOP within ±3 mmHg which seems to be clinically acceptable but need to be carefully interpreted. However, the IOP agreement between CST and GAT in eyes with MCD was poor. Considering the thinner CCT and abnormal biomechanics in MCD, the CST may over-correct the IOP in thinner and softer corneas.
The CCT provided by CST showed a strong correlation with the one from the USP in healthy corneas, with a small overestimated bias and a narrow 95% LoA. Although Yu et al. reported a contrary result of underestimated CCT in CST compared to the USP, the 95% LoA in their study was similarly narrow [31]. The CCT measured by CST is based on the Scheimpflug imaging principle. In normal corneas, the CCT measured by ultrasound pachymetry and Scheimpflug topography are significantly correlated and the magnitude of difference is small [32]. Considering the high repeatability of CCT measurement in CST [16], it may be interchangeable in normal eyes in most clinical applications. However, Unlike USP that uses short electrical pulses [33], CST measures the CCT by the initial state of corneal cross-section diagram based on optical technologies that might be influenced by the accumulated deposits in corneal dystrophy. In eyes with corneal opacity, the Scheimpflug topography may underestimate the CCT due to the assumption of the clear cornea interpolation as the posterior surface [34]. In our study, similar results were observed that the CCT was underestimated by CST in eyes with LCD that showing corneal opacity in the Scheimpflug image. The linear and other shaped deposits progress particularly within central corneal stroma in LCD [6], which could make a great difference on the CCT measured by CST. Although the biases of CCT were small between Corvis ST and ultrasound pachymetry in eyes with GCD and MCD, the magnitude of difference was large (−71.6 to 73.6 μm) in MCD. While in eyes with GCD, the magnitude of difference was relatively small (−43.5 to 34.8 μm) which may result from the clear intervening stromal areas between the deposits. Therefore, clinicians should be careful to interpret the CCT provided by Corvis ST in patients with corneal dystrophy, especially those with corneal opacity. As to the corneal biomechanical properties provided by CST, the biomechanical values are based on the identified anterior surface of the cornea. We believe that the biomechanical readings are still reliable in eyes with corneal opacity while further studies might be necessary to verify our assumption.
There are some limitations in the present study. The corneal dystrophy was diagnosed based on the patients’ clinical corneal appearance and their family history. Although the International Committee for Classification of Corneal Dystrophy (IC3D) developed a detailed classification system for corneal dystrophies based on genetic diagnosis6, this preliminary study did not perform genetic diagnosis due to the relatively small sample size and the limited acceptance of taking genetic test in patients with corneal dystrophy. Further study with a large sample size and application of genetic diagnosis is necessary to provide more information on corneal biomechanical changes in corneal dystrophies and their subtypes. In addition, the CCT measurement in CST is derived from corneal apex while it is obtained by USP based on pupil centre, and the topical anaesthetic may have a small influence on CCT measurement [35].
In conclusion, corneal biomechanics was altered in eyes with corneal dystrophy (GCD, LCD and MCD), including a decreased SPA1 and increased A1DA and CBI. The corneas with MCD showed a higher A1DA after CCT adjustment compared to GCD or LCD. CST showed overestimated IOP that needs to be carefully interpreted. The CCT obtained by CST and USP may be interchangeable in normal eyes but not in those with corneal dystrophy.
Summary
What was known before
-
Corneal hysteresis and corneal resistance factor are reduced in Fuchs’ endothelial dystrophy Biomechanical alterations in corneal dystrophies can be evaluated using Corvis ST tonometer.
What this study adds
-
Macular corneal dystrophy had a higher A1DA compared to granular and lattice ones IOP obtained by Corvis ST showed overestimated bias to Goldmann applanation tonometer CCT in healthy eyes showed good agreement between Corvis ST and ultrasound pachymeter.
References
Ali NQ, Patel DV, McGhee CN. Biomechanical responses of healthy and keratoconic corneas measured using a noncontact scheimpflug-based tonometer. Invest Ophthalmol Vis Sci. 2014;55:3651–9.
Winkler M, Shoa G, Xie Y, Petsche SJ, Pinsky PM, Juhasz T, et al. Three-dimensional distribution of transverse collagen fibers in the anterior human corneal stroma. Invest Ophthalmol Vis Sci. 2013;54:7293–301.
Shen Y, Chen Z, Knorz MC, Li M, Zhao J, Zhou X. Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J Refract Surg. 2014;30:310–8.
Vinciguerra R, Romano V, Arbabi EM, Brunner M, Willoughby CE, Batterbury M, et al. In vivo early corneal biomechanical changes after corneal cross-linking in patients with progressive keratoconus. J Refract Surg. 2017;33:840–6.
Perez-Rico C, Gutierrez-Ortiz C, Gonzalez-Mesa A, Zandueta AM, Moreno-Salgueiro A, Germain F. Effect of diabetes mellitus on Corvis ST measurement process. Acta Ophthalmol. 2015;93:e193–8.
Weiss JS, Moller HU, Aldave AJ, Seitz B, Bredrup C, Kivela T, et al. IC3D classification of corneal dystrophies–edition 2. Cornea. 2015;34:117–59.
Musch DC, Niziol LM, Stein JD, Kamyar RM, Sugar A. Prevalence of corneal dystrophies in the United States: estimates from claims data. Invest Ophthalmol Vis Sci. 2011;52:6959–63.
Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–62.
Hong J, Xu J, Wei A, Deng SX, Cui X, Yu X, et al. A new tonometer–the Corvis ST tonometer: clinical comparison with noncontact and Goldmann applanation tonometers. Invest Ophthalmol Vis Sci. 2013;54:659–65.
Bak-Nielsen S, Pedersen IB, Ivarsen A, Hjortdal J. Repeatability, reproducibility, and age dependency of dynamic Scheimpflug-based pneumotonometer and its correlation with a dynamic bidirectional pneumotonometry device. Cornea. 2015;34:71–7.
Tejwani S, Shetty R, Kurien M, Dinakaran S, Ghosh A, Sinha Roy A. Biomechanics of the cornea evaluated by spectral analysis of waveforms from ocular response analyzer and Corvis-ST. PLoS ONE. 2014;9:e97591.
Clemmensen K, Hjortdal J. Intraocular pressure and corneal biomechanics in Fuchs’ endothelial dystrophy and after posterior lamellar keratoplasty. Acta Ophthalmol. 2014;92:350–4.
Bhikoo R, Vellara H, McKelvie J, McGhee CN, Patel DV. The effect of abnormal stromal protein on the biomechanical properties of the cornea. Clin Exp Optom. 2017;100:729–31.
Roberts CJ, Mahmoud AM, Bons JP, Hossain A, Elsheikh A, Vinciguerra R, et al. Introduction of two novel stiffness parameters and interpretation of air puff-induced biomechanical deformation parameters with a dynamic scheimpflug analyzer. J Refract Surg. 2017;33:266–73.
Vinciguerra R, Ambrosio R Jr, Elsheikh A, Roberts CJ, Lopes B, Morenghi E, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32:803–10.
Wang W, He M, He H, Zhang C, Jin H, Zhong X. Corneal biomechanical metrics of healthy Chinese adults using Corvis ST. Cont Lens Anterior Eye. 2017;40:97–103.
Edmund C. Corneal elasticity and ocular rigidity in normal and keratoconic eyes. Acta Ophthalmol (Copenh). 1988;66:134–40.
Salvetat ML, Zeppieri M, Tosoni C, Felletti M, Grasso L, Brusini P. Corneal deformation parameters provided by the Corvis-ST Pachy-Tonometer in healthy subjects and glaucoma patients. J Glaucoma. 2015;24:568–74.
Kataria P, Padmanabhan P, Gopalakrishnan A, Padmanaban V, Mahadik S, Ambrosio R Jr. Accuracy of Scheimpflug-derived corneal biomechanical and tomographic indices for detecting subclinical and mild keratectasia in a South Asian population. J Cataract Refract Surg. 2019;45:328–36.
Rabinowitz YS. The genetics of keratoconus. Ophthalmol Clin North Am. 2003;16:607–20.
Magalhaes OA, Kronbauer CL, Muller EG, Sanvicente CT. Update and review of Urrets-Zavalia syndrome. Arq Bras Oftalmol. 2016;79:202–4.
Espana EM, Ioannidis A, Tello C, Liebmann JM, Foster P, Ritch R. Urrets-Zavalia syndrome as a complication of argon laser peripheral iridoplasty. Br J Ophthalmol. 2007;91:427–9.
Davies PD, Ruben M. The paretic pupil: its incidence and aetiology after keratoplasty for keratoconus. Br J Ophthalmol. 1975;59:223–8.
Chan TCY, Wang YM, Yu M, Jhanji V. Comparison of corneal tomography and a new combined tomographic biomechanical index in subclinical keratoconus. J Refract Surg. 2018;34:616–21.
Lanza M, Cennamo M, Iaccarino S, Romano V, Bifani M, Irregolare C, et al. Evaluation of corneal deformation analyzed with a Scheimpflug based device. Cont Lens Anterior Eye. 2015;38:89–93.
Aggarwal S, Peck T, Golen J, Karcioglu ZA. Macular corneal dystrophy: a review. Surv Ophthalmol. 2018;63:609–17.
Reznicek L, Muth D, Kampik A, Neubauer AS, Hirneiss C. Evaluation of a novel Scheimpflug-based non-contact tonometer in healthy subjects and patients with ocular hypertension and glaucoma. Br J Ophthalmol. 2013;97:1410–4.
Smedowski A, Weglarz B, Tarnawska D, Kaarniranta K, Wylegala E. Comparison of three intraocular pressure measurement methods including biomechanical properties of the cornea. Invest Ophthalmol Vis Sci. 2014;55:666–73.
Lam A, Chen D, Chiu R, Chui WS. Comparison of IOP measurements between ORA and GAT in normal Chinese. Optom Vis Sci. 2007;84:909–14.
Carbonaro F, Andrew T, Mackey DA, Spector TD, Hammond CJ. Comparison of three methods of intraocular pressure measurement and their relation to central corneal thickness. Eye (Lond). 2010;24:1165–70.
Yu A, Zhao W, Savini G, Huang Z, Bao F, Lu W, et al. Evaluation of central corneal thickness using corneal dynamic scheimpflug analyzer Corvis ST and comparison with pentacam rotating scheimpflug system and ultrasound pachymetry in normal eyes. J Ophthalmol. 2015;2015:767012.
MeenakshiSundaram S, Sufi AR, Prajna NV, Keenan JD. Comparison of in vivo confocal microscopy, ultrasonic pachymetry, and scheimpflug topography for measuring central corneal thickness. JAMA Ophthalmol. 2016;134:1057–9.
Calvo-Sanz JA, Ruiz-Alcocer J, Sanchez-Tena MA. Accuracy of Cirrus HD-OCT and Topcon SP-3000P for measuring central corneal thickness. J Optom. 2018;11:192–7.
Kanellopoulos AJ, Asimellis G. Clinical correlation between placido, scheimpflug and LED color reflection topographies in imaging of a scarred cornea. Case Rep Ophthalmol. 2014;5:311–7.
Nam SM, Lee HK, Kim EK, Seo KY. Comparison of corneal thickness after the instillation of topical anesthetics: proparacaine versus oxybuprocaine. Cornea. 2006;25:51–4.
Funding
This work was supported by National Natural Science Foundation of China (grant No. 81100636).
Author information
Authors and Affiliations
Contributions
TS and YW were responsible for designing the study. MW, JH and YW were responsible for the subject’s enrolment and data collection. MW, JH and XW contributed to the data analysis and interpretation. MW and JH drafted the manuscript. MW, JH, XW, TS and YW edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, M., Han, J., Wang, X. et al. The alterations of corneal biomechanics in adult patients with corneal dystrophy. Eye 37, 492–500 (2023). https://doi.org/10.1038/s41433-022-01965-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-01965-z