Abstract
Introduction
As gonococcal infections continue to increase, we wanted to review the number and clinical course of recent ocular gonococcal cases presenting to ophthalmology departments in NHS Greater Glasgow and Clyde.
Methods
A 5-year retrospective review of adult ocular gonococcal cases, where the diagnosis of Neisseria gonorrhoeae was made on microbiological culture, was undertaken.
Results
Fifteen cases were identified (80% male). Average age was 26 years (range 17–42; median 24). Most common presenting features included purulent discharge (14/15; 93%), haemorrhagic conjunctivitis (10/15; 67%) and pre-septal cellulitis (9/15; 60%). Corneal involvement was documented in 5 (33%), with marginal ulceration in 1 (7%) but none had corneal perforation. Most common systemic treatment was IV ceftriaxone, alone or in combination with another antibiotic (6/15; 40%), followed by IM ceftriaxone, alone or in combination with another antibiotic (5/15; 33%). Median time from presentation to treatment was 1 day (0–23). All patients were referred or recommended to attend sexual health services. Seven patients (47%) attended and received complete sexually transmitted infection (STI) testing and contact tracing: 3 patients had systemic treatment initiated or changed at this visit and 1 patient had concurrent syphilis identified.
Conclusions
This series confirms purulent conjunctivitis and cellulitis as the main presenting features of ocular gonococcal infection requiring hospital review. Early identification with appropriate systemic antibiotic treatment avoided corneal melting in this cohort. As concurrent STIs were identified and/or treatments changed in 4/7 (57%) following sexual health review, we recommend a shared care approach between ophthalmology, microbiology and sexual health services to effectively address all management issues.
Similar content being viewed by others
Introduction
The incidence of sexually transmitted infections (STIs) has continued to increase across the UK in the past 5 years, including new gonococcal infections increasing from 2346 to 3776 (61%) in Scotland and from 41,382 to 70,936 (71%) in England between 2015 and 2019 [1,2,3,4]. Gonococcal infection, caused by the gram-negative diplococcus Neisseria gonorrhoeae, typically manifests as a genitourinary tract infection [5]. However, N. gonorrhoeae can also infect the mucous membranes of the mouth, throat and eye [6]. Transmission to the eye normally occurs through autoinoculation but can also be transferred by direct exposure to infected urinary or genital secretions [7, 8]. Ocular gonococcal infection frequently causes an intense, purulent conjunctivitis often associated with lid swelling and pain [8,9,10]. Corneal involvement is reported to be common, including sub-epithelial or stromal infiltrates, discrete corneal oedema, severe keratitis and peripheral corneal ulceration [6, 8,9,10,11]. Ulceration can subsequently progress rapidly to corneal melting and perforation (traditionally reported as within 48 h) [12, 13]. Awareness of this visual impairment highlights the need for prompt and effective management, particularly as the final clinical outcome has been shown to relate to disease severity at initiation of appropriate systemic therapy [8].
The rising rates of resistance towards penicillin, tetracyclines and fluoroquinolones and the progressive decrease in susceptibility of N. gonorrhoeae to azithromycin and cephalosporins remains a significant public health concern [9]. Emergence of such antibiotic-resistant strains has resulted in changes in recent management guidelines. For example, the Centre for Disease Control (CDC) current recommendations for ocular gonorrhoea are ceftriaxone 1 g IM in a single dose plus azithromycin 1 g orally in a single dose [14]. However, the British Association for Sexual Health and HIV (BASHH) has reported growing numbers of azithromycin-resistant N. gonorrhoeae strains [15]. In 2018 the UK guidelines for treating gonococcal disease changed from dual therapy to ceftriaxone monotherapy [16]. Systemic treatment should be administered as soon as N. gonorrhoeae infection has been confirmed, for the reasons previously mentioned regarding rapid disease progression. Adjuvant topical therapies can include chloramphenicol or ofloxacin but the mainstay treatment remains systemic, particularly as 30–40% of N. gonorrhoeae strains are resistant to these ocular medications [17].
Ocular gonococcal infection has traditionally been identified by routine microbiological culture from purulent conjunctivitis. More recently, detection by nucleic acid amplification tests (NAATs), including PCR, have been shown to be quick and accurate [18]. Given the mode of transmission of ocular gonococcal infection, it is likely that patients have been in contact with genitourinary gonococcus and potentially be at risk of having other co-existing STIs [14]. Best management should involve specialist sexual health input to perform genitourinary testing, partner notification and provide patient education on safe sexual practices to prevent re-infection [16, 19]. In the West of Scotland region, Sandyford Sexual Health provides specialist sexual health services to the approximately 1 million population of NHS Greater Glasgow and Clyde (NHSGGC). The Clinical Microbiology service for NHSGGC is delivered from two laboratories based at Glasgow Royal Infirmary and Queen Elizabeth University Hospital. Each laboratory is UKAS (United Kingdom Accreditation Service) accredited providing a full and comprehensive microbiology diagnostic service. The microbiology investigation of bacterial eye infections, which includes the target organism N. gonorrhoeae, is performed in accordance with the UK Standards for Microbiology Investigations, Public Health England (PHE) guidance [19]. For isolation of N. gonorrhoeae, the standard incubation time is 40–48 h, however culture plates are reviewed for growth every day in the laboratory. As with any clinically significant isolate, all laboratory diagnosed or microbiologically confirmed cases of gonococcal infection are reported back to the clinician requesting the test or the relevant medical practitioner responsible for the patient’s care. As a result, Sandyford Sexual Health Services are currently only notified of cases of ocular gonococcal infection through self-presentation or via direct communication by a referring ophthalmologist.
In this retrospective study, we wished to ascertain whether the rise in cases of genitourinary gonococcal infection over the last 5 years has translated to an increase in cases of adult ocular gonococcal infections detected in NHSGGC, to establish the clinical course of these infections, and to determine what proportion of patients presenting with ocular infection were subsequently assessed by Sandyford Sexual Health Services.
Methods
This study was discussed with the West of Scotland Ethics Committee and as it was deemed to be an audit of established clinical practice, no additional ethical permissions were required. Caldicott approval for this study was granted by NHSGGC Information Governance in July 2020. The principles of the Declaration of Helsinki were maintained throughout this audit.
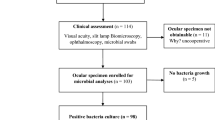
In Greater Glasgow and Clyde, ocular gonococcal infections are currently identified through routine microbiological culture; gonorrhoea detection is not included in our local in-house eye PCR swab testing. Cases of ocular gonococcal infection were identified retrospectively from our laboratory information management system (Telepath) over the 5-year study period from 1 June 2015 to 31 May 2020. Data on specimens processed by the two Microbiology laboratories that serve NHSGGC (the Glasgow Royal Infirmary and the Queen Elizabeth University Hospital) were reviewed. Specimens included any sample labelled as an eye swab, conjunctival swab/ fluid or corneal scraping. From the dataset of positive results, patient demographic and clinical information was then gathered from electronic patient records. Demographic information included age and sex only. Clinical information included presenting features, corneal involvement, treatment received, onward referral to sexual health services, sexual health screen and whether any contact tracing had been carried out.
Results
Patient numbers
There were 15 cases of adult ocular gonococcal infection identified by the ophthalmology departments in NHSGGC between 1 June 2015 and 31 May 2020. Of the 15 cases, 80% were male and 20% were female. The average age was 26 years (median age 24, range 17–42). The number of cases of ocular gonococcal infection per 12-month period ranged from 0 to 6 with an overall upward trend. The highest number of cases were seen in more recent years (6 cases in June 2018–May 2019; 5 cases in June 2019–May 2020). A similar increasing trend was found in the number of overall cases of gonococcal infection in NHSGGC, which increased from 804 cases in 2015 to 1242 cases in 2019 (see Fig. 1).
Clinical presentation
Ophthalmic case records were available for all 15 patients. All patients were referred to the secondary care ophthalmology acute referral clinic either by an optometrist, a general practitioner or an accident and emergency physician. All cases were unilateral. The most common presenting features were purulent discharge (14/15; 93%), haemorrhagic conjunctivitis (10/15; 67%) and pre-septal cellulitis (9/15; 60%). Four patients required inpatient admissions for severe pre-septal cellulitis and 2 underwent CT scanning to rule out orbital involvement.
Mild corneal involvement was documented in 5 (33%) of the cases: 2 patients had punctate epithelial staining; 1 had stromal inflammation; 1 had inflammatory keratitis; and 1 had early marginal ulceration. No patient had significant corneal melting or corneal perforation (see Fig. 2).
Treatment
In all, 14 patients (93.3%) were documented as having received some form of systemic antibiotics (1 patient was uncontactable, so messages were left with their GP). The median time from presentation to commencement of systemic therapy was 1 day (range 0–23). The patient who had a 23 day delay in treatment was on holiday abroad when the results became available and was not contactable until return.
Choice of systemic treatment varied in this cohort of patients. Those with a suspected diagnosis of pre-septal cellulitis were treated empirically as per the local orbital cellulitis protocol (6 patients). Those with severe cellulitis were admitted and treated with IV antibiotics (4 patients). All others were treated on receipt of positive swab results. The median time from obtaining the sample to receiving the swab result was 48 h (range <24–120 h). As with any clinically significant isolate, in addition to the report being issued on the electronic records, the interim culture results were verbally reported to the requesting clinician. The route of antibiotic delivery (IV or IM) was based on swab results and the advice of the reporting microbiologist or an infectious diseases specialist. The most common systemic treatment was IV ceftriaxone (6/15; 40%), which was given as monotherapy in 1 case and in combination with other antibiotics in 5 cases. The second most common treatment was IM ceftriaxone (5/15; 33%), which was given as monotherapy in 3 cases, in combination with azithromycin in 1 case and in combination with oral co-amoxiclav in 1 case (in this patient oral co-amoxiclav had already been started for pre-septal cellulitis prior to knowledge of positive ocular N. gonorrhoeae swab). One patient was treated with a combination of IV cefotaxime and oral azithromycin; one with oral ciprofloxacin (this was on the advice of an infectious diseases specialist, and this particular N. Gonorrhoeae strain was sensitive); one with oral co-amoxiclav for a presumed pre-septal cellulitis (who then failed to attend follow-up for further treatment); and in one case there was no known systemic treatment given as they failed to attend for treatment once the diagnosis was known. Additionally, all patients were treated with either topical chloramphenicol or topical ofloxacin.
All N. gonorrhoeae strains that were identified in this series were sensitive to the recommended treatment of ceftriaxone. The most common resistance was to tetracycline (7/15; 47%) followed by ciprofloxacin (3/15; 20%).
Sexual health services
All 15 patients in this series were either referred directly or recommended to attend sexual health services; 7 patients (47%) attended and underwent further STI testing and completed contact tracing. The median time between referral and attendance at sexual health services for these 7 patients was 3 days (range 0–12). Two patients had concurrent STI infections identified, including gonococcal throat infection and genitourinary syphilis. Three patients had their systemic treatment changed at this visit. There were 8 patients (53%) who did not attend the sexual health services. Of these, a letter was written to the GP for 4 patients, the sexual health officer closed the file of 3 patients as they were uncontactable but received appropriate systemic therapy and 1 patient was not contactable following notification of a diagnosis. These 8 patients who did not attend, did not have contact tracing carried out and were not screened for concurrent STIs (see Table 1 for details).
Discussion
As the number of cases of genitourinary gonococcal infection increases, we would expect a similar rise in those with ocular involvement or additional co-infections [20]. However, a report from Melbourne in 2017 did not identify a corresponding rise in cases of ocular gonococcal infection [21]. The authors looked at cases of ocular infection over a period of 7 years (2000–2017) and found there had been 24 cases in total, with between 0 and 3 per year over the 7 years. This rate was not in keeping with the increasing numbers in overall N. gonorrhoeae infections that was seen in the same population over that time period. It was hypothesised that perhaps the practice of good hand hygiene was the reason for this, reducing the rate of self-inoculation via the hands. In contrast to this, a 2015 study from Dublin found an increase in cases of ocular gonococcal infection among young adults that seemed to correspond to the rising rates of N. gonorrhoeae infection in the same population [11]. They identified 14 adult cases of ocular gonococcal infection over 3 years (2011–2013), presenting predominantly in young adult males, the demographic group in which the overall rates of N. gonorrhoeae infection were rising in Ireland during the same period. These findings are similar to our study and would be in keeping with what we would expect, given the rapidly increasing rates of gonococcal infection. Our study suggests that as the rates of gonorrhoea in NHSGGC have increased, so too has the number of patients presenting with ocular gonococcal symptoms. The demographics of our patients in this series are consistent with the national data for gonococcal infection in general, with the disease being more prevalent in men, and in younger age groups [4].
In our series, the clinical presentation was largely that of haemorrhagic conjunctivitis, purulent discharge, and lid oedema consistent with pre-septal cellulitis. This is in keeping with the published literature [10, 11, 22,23,24]. However, corneal involvement was observed less commonly in our patients, and was mild when present. Traditional teaching states that N. gonorrhoeae can cause corneal perforation within 24–48 h, and there are documented cases of ocular infection causing corneal perforation in the literature [9, 12, 13, 18, 23,24,25,26,27]. These include 6 single case reports and 2 case series; one published in 1987 including 5 patients [23] and one published in 2009 including 4 adult patients [12]. However, all these cases were either delayed in the presentation, diagnosis or had additional complicating factors such as history of corneal trauma [9] or recent chemical injury [13]. Even with these delays and/or complications, the time from onset of symptoms to corneal perforation was between 7 days and 3 weeks in all but one case report; that patient in question was a Malay man who was a contact lens wearer and used a traditional method of using his urine to wash his eye out [25]. In our cohort of patients there was minimal delay between time of presentation and commencement of systemic treatment. The findings in the published literature, combined with our observations in this study, would suggest that it would be rare for corneal perforation to occur as rapidly as we may have previously been thought if patients present in a timely fashion, obtain the correct diagnosis and commence prompt appropriate systemic treatment [8]. The involvement of sexual health services in the care of these patients is extremely valuable. Contact tracing is important for public health reasons and screening for the detection of any concurrent disease is necessary in these patients to ensure they receive adequate treatment. However, patients are often lost to follow-up following referral from ophthalmology to sexual health services [28]. In 2009, the Sandyford Sexual Health clinic set up a shared care pathway with the ophthalmology department in NHSGGC to address these issues of non-attendance and poor engagement for patients with ocular Chlamydia trachomatis (OC) [29]. Briefly, Sandyford provides a designated sexual health advisor who receives copies of positive OC viral eye swab results and act as a central point of contact to coordinate ongoing or further treatment. This includes contacting any patients who have not engaged with their planned appointment. This shared care approach more than doubled the subsequent attendance to the Sandyford Sexual Health clinic from 25% to 57% over a 10-year period [28, 29].
Despite such a system being established for STIs (OC) identified from ocular virology testing, there is currently no shared care pathway of communication between the microbiology team and the sexual health services in Glasgow. This is reflected in the low attendance rates at sexual health services of less than 50% in our cohort of patients. A high failure to attend sexual health services was noted in this study (8 patients; 53%). This means 53% of patients identified with ocular gonococcal infection by ophthalmologists in this 5-year period did not undergo contact tracing, were not investigated for concurrent STIs and did not necessarily have the most effective treatment for their condition. This point is further demonstrated, as in those that did attend sexual health services, 3 patients had treatment altered or even initiated for the first time. Additionally, a concurrent STI (syphilis) was picked up in 1 patient that may otherwise have gone undiagnosed, exposing the patient and potentially others to further harm. Comprehensive contact tracing was carried out in all 7 patients who attended sexual health services. The median time between patient notification of their swab result to attendance at sexual health services of 3 days is somewhat reassuring, however, patients being delayed for longer periods (up to 12 days in our cohort) could potentially have concurrent infections that they are unaware of, exposing any partners to possible harm. This only serves to highlight the importance of prompt referral to these specialist services for optimal treatment and the need for ophthalmologists to refer and strongly encourage patients presenting with any ocular STIs to engage with their management. We would suggest that management of patients with ocular gonococcal infection would be improved via the adoption of a shared care approach similar to that in place in Glasgow for OC [29].
Study limitations and suggestions for future work
This study is modest in size and was limited to Greater Glasgow and Clyde (NHSGGC). Patients were identified by a retrospective review of culture proven/microbiologically confirmed cases of N. gonorrhoeae ocular infection. Due to reconfiguration of the microbiology laboratory services in NHSGGC and the subsequent unification of the laboratory information management systems, only 5 years of historic data were accessible for analysis. Following this audit, Sandyford Sexual Health Services identified just 6 eye swab tests from patients with positive gonococcal NAAT genitourinary results over this 5-year period (all of which had negative ocular swabs). This may suggest that patients do not typically co-present to sexual health services with conjunctivitis and genital discharge. Additionally, there have been published cases demonstrating that NAATs have detected ocular N. gonorrhoeae where microbiology culture has been negative [11, 18]. Integration of NAAT testing for both ocular and genitourinary testing could identify more ocular gonococcal cases in the future.
This retrospective audit may underestimate the true number of ocular gonococcal cases in this 5-year period, as not every case of conjunctivitis is routinely swabbed for microbiological investigation. It is possible that more cases remain undetected due to the availability of over-the-counter topical antibiotics and high level of self-management of conjunctivitis (without microbiological testing) in the community. Equally, patients with genitourinary N. gonorrhoeae infection may have been identified and treated systemically by sexual health services or in primary care, and their eyes may not have been formally examined or tested for ocular gonococcal infection.
Conclusion
This series confirms purulent conjunctivitis and cellulitis as common presenting features of ocular N. gonorrhoeae infection, yet contradicts the traditional teaching of corneal perforation being common. Early identification with appropriate systemic antibiotic treatment avoided corneal melting in this cohort, which appears rare with modern interventions. A high failure to attend sexual health services was noted in this study (8 patients; 53%). As concurrent STIs were identified and/or treatments changed in 4/7 patients (57%) following sexual health review, we recommend a shared care approach between ophthalmology, microbiology and sexual health services to effectively address all management issues.
Summary
What was known before:
-
The rates of Neisseria gonorrhoeae infection in the UK population have increased over the past 5 years (61% increase from 2346 to 3776 cases in Scotland alone)
-
Traditional teaching advises ocular gonococcal infection often involves the cornea and can cause corneal melting and perforation within 48 h
What this study adds:
-
We identified 15 cases of ocular gonococcal infection in this 5-year period, with an upward trend in more recent years
-
The most common clinical presentations were purulent discharge (14/15; 93%), haemorrhagic conjunctivitis (10/15; 67%) and pre-septal cellulitis (9/15; 60%)
-
With appropriate timely systemic treatment, the risk of corneal perforation was low (0% in our series of 15 patients)
-
We recommend a shared care approach between microbiology, ophthalmology and the sexual health services to ensure optimal care for patients with ocular gonococcal infection
References
Health Protection Scotland. Diagnosis of STIs continues to rise in Scotland. 2019. https://www.hps.scot.nhs.uk/publications/hps-weekly-report/volume-53/issue-21/diagnoses-of-stis-continue-to-rise-in-scotland/#:~:text=Two%20reports%20published%20by%20Health%20Protection%20Scotland%20%28HPS%29,2018%2C%20specifically%20genital%20chlamydia%2C%20gonorrhoea%20and%20infectious%20syphilis. Accessed 18 September 2020.
Public Health England. Heath matters: preventing STIs. 2019. https://www.gov.uk/government/publications/health-matters-preventing-stis/health-matters-preventing-stis. Accessed 18 September 2020.
Public Health England. STI diagnoses and rates in England by gender, 2010–2019. 2020. https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables. Accessed 18 September 2020.
Health Protection Scotland. Genital chlamydia and gonorrhoea infections in Scotland: laboratory diagnosis in Scotland 2010–2019. https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3073/documents/1_genital-chlamydia-gonorrhoea-scotland-2010-2019-summary.pdf. Accessed 18 September 2020.
Miller K.E. Diagnosis and treatment of Neisseria gonorrhoeae infections. Am Fam Physician. 2006;73:1779–784.
Centers for Disease Control and Prevention. Gonorrhoea – CDC Factsheet. 2019. https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm. Accessed 18 September 2020.
Stenberg K, Mardh PA. Chlamydial conjunctivitis in neonates and adults. History, clinical findings, and follow-up. Acta Ophthalmol 1990;68:651–7.
Lee JS, Choi HY, Lee JE, Lee SH, Oum BS. Gonococcal keratoconjunctivitis in adults. Eye 2002;16:646–9.
McElnea E, Stapleton P, Kahn S, Stokes J, Higgins G. Challenges in the management of Neisseria gonorrhoeae keratitis. Int Ophthalmol 2015;35:135–40.
Wan WL, Farkas GC, May WN, Robin JB. The clinical characteristics and course of adult gonococcal conjunctivitis. Am J Ophthalmol 1986;102:575–83.
McAnena L, Knowels SJ, Curry A, Cassidy L. Prevelance of gonococcal conjunctivitis in adults and neonates. Eye 2015;29:875–80.
Kawashima M, Kawakita T, Den S, Tomita M, Shimazaki J. Surgical management of corneal perforation secondary to gonococcal keratoconjunctivitis. Eye 2009;23:339–44.
Tipple C, Smith A, Bakowska E, Corbett MC. Corneal perforation requiring corneal grafting: a rare complication of gonococcal eye infection. Sex Transm Infect 2010;86:447–8.
Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. 2015. https://www.cdc.gov/std/tg2015/gonorrhea.htm. Accessed 24 September 2020.
BASHH. Coordinated response to restrain further spread of azithromycin resistant gonorrhoea. 2016. https://www.bashh.org/news/news/bashh-calls-for-coordinated-response-to-restrain-further-spread-of-azithromycin-resistant-gonorrhoea/. Accessed 24 September 2020.
Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. 2018 National UK guideline for management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020;31:4–15.
Day AC, Ramkissoon YD, George S, Corbett MC. Don’t forget Gonococcus! Eye 2006;20:1400–2.
Sevilla N, Martin S, Serr-Pladevall J, Kirkegaard E, Bisbe L, Puig JJ. Delay in diagnosis resulting in corneal perforation: nucleic acid amplification tests for a rapid identification of ocular Neisseria gonorrhoeae infection. Sex Transm Infect 2020;96:562.
Public Health England. SMIB2: investigations of bacterial eye infections. 2017. https://www.gov.uk/government/publications/smi-b-2-investigation-of-eye-swabs-and-canalicular-pus. Accessed 6 December 2020.
Nusbaum MRH, Wallace RR, Slatt LM, Kondrad EC. Sexually transmitted infections and increased risk of co-infection with human immunodeficiency virus. J Am Osteopath Assoc 2004;104:527–35.
Rothschild P, Sherwin J, Chen Y, Wells K, Crock C. Has the increasing incidence of chlamydia and gonorrhoea resulted in increased chlamydial and gonococcal conjunctivitis presentations? Results from Melbourne, Australia, from 2000 to 2017. Clin Exp Ophthalmol 2019;47:289–91.
Hegde V, Smith G, Choi J, Pagliarini S. A case of gonococcal kerato-conjunctivitis mimicking orbital cellulitis. Acta Ophthalmol Scand 2005;83:511–2.
Ullman S, Roussel TJ, Culbertson WW, Forster RK, Alfonso E, Mendelsohn AD, et al. Neisseria gonorrhoeae keratoconjunctivitis. Ophthalmology 1987;94:525–31.
Duke-Elder S, editor. System of ophthalmology. Vol. 8. Part 1. Diseases of the outer eye. CV Mosby Co.; 1965. p. 167–74.
Tong L, Tan DT, Abańo JM, Lim L. Deep anterior lamellar keratoplasty in a patient with descemetocele following gonococcal keratitis. Am J Ophthalmol 2004;138:506–7.
Samira N, Bani AP, Susiyanti M. Rare case of bilateral perforated corneal ulcer due to gonococcal infection, managed with temporary periosteal graft. BMJ Case Rep. 2016. https://doi.org/10.1136/bcr-2015-213547
Bastion ML, Prakash K, Siow YC, Loh SS. Bilateral corneal perforation in a sexually active adult male with gonococcal conjunctivitis. Med J Malaysia 2006;61:366–8.
Lockington D, MacDonald R, King S, Weir C, Winter A, Aitken C. Multiplex PCR testing requires a robust multi-disciplinary strategy to effectively manage identified cases of chlamydial conjunctivitis. Scott Med J 2013;58:77–82.
Shah M, Gishkori S, Edington M, King S, Winter A, Lockington D. Ten-year review of a shared care approach in the management of ocular Chlamydia trachomatis infections. Eye . 2021;35:1614–9. https://doi.org/10.1038/s41433-020-01128-y.
Acknowledgements
A version of this paper was an oral presentation at the UK Bowman Club meeting in March 2021 and a poster at the Virtual Annual Congress of the Royal College of Ophthalmologists in May 2021.
Author information
Authors and Affiliations
Contributions
D.L. had the original idea. L.B. and M.S. performed the data collection, initial literature review and drafted the article. L.C., A.W. and D.L. provided direction and senior support, additional literature input and revised the article into the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Permission for image publication: Granted and consent obtained
Rights and permissions
About this article
Cite this article
Butler, L., Shah, M., Cottom, L. et al. Five-year review of ocular Neisseria gonorrhoeae infections presenting to ophthalmology departments in Greater Glasgow & Clyde, Scotland. Eye 36, 1442–1447 (2022). https://doi.org/10.1038/s41433-021-01658-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01658-z