Abstract
Objective
To evaluate the surgical outcomes of patients with hereditary transthyretin amyloidosis (TTR-FAP) who underwent Ahmed glaucoma valve (AGV) implantation.
Methods
A retrospective cohort study was performed on patients with a diagnosis of TTR-FAP secondary glaucoma, who underwent AGV implantation in our department, between November 2010 and July 2019. The cumulative probability of treatment success was measured with Kaplan-Meier survival analysis. The primary outcome was success, defined as intraocular pressure (IOP) ≥ 6 mmHg and ≤21 mmHg with or without medication, with no need for further glaucoma surgery and without loss of light perception at last follow-up. Secondary outcomes were postoperative IOP, number of IOP-lowering medications, and rates of complications.
Results
The study included 114 eyes of 87 patients. The mean follow-up duration was 3.81 ± 2.11 years (y) [range, 1.00–8.28 y]. Compared to the preoperative values, the mean IOP was reduced from 28.20 ± 7.01 to 12.87 ± 3.76 mmHg at the final visit (p < 0.001), with a reduction in the number of medications from 3.89 ± 0.66 to 1.86 ± 1.43 (p < 0.001). Early and late postoperative complications occurred in 20 (17.09%) and 9 (7.89%) eyes, respectively. Kaplan–Meier analysis indicated probabilities of success of 0.98 at 1 y, 0.97 at 2 y, 0.95 at 3 y, 0.89 at 4 y, 0.77 at 5 y and 0.72 at 6 y. The linear correlation analysis showed a correlation between some characteristics of the natural history of TTR-FAP patients and AGV implantation success.
Conclusion
Although glaucoma in TTR-FAP patients is very difficult to manage, AGV implantation is an effective and relatively safe procedure.
Similar content being viewed by others
Introduction
Hereditary transthyretin amyloidosis, also known as transthyretin-related familial amyloidotic polyneuropathy (TTR-FAP) was described in Portugal in 1939 and first published in 1952 by Corino Andrade [1]. In 1968 new cases were identified in Japan and in 1976 in Sweden. Nowadays it can be found worldwide [2]. It is a progressive neurodegenerative disease, inherited in an autosomal dominant pattern, characterized by the accumulation of mutant amyloidogenic TTR in peripheral nerves and organs, including the eye [3].
Relatively to ocular involvement, TTR-FAP patients can present with vitreous opacities, secondary open-angle glaucoma, abnormal conjunctival vessels, keratoconjunctivitis sicca, loss of corneal sensitivity and neurotrophic corneal ulcers, anterior capsule opacity of the lens, retinal vascular changes, pupillary light-near dissociation, irregular pupil and optic neuropathy [4,5,6]. The pathophysiology of glaucoma in these patients is still poorly understood. Some defend extensive amyloid deposition in the intertrabecular space and Schlemm’s canal conditioning the flow of aqueous humour and deposition of perivascular amyloid in conjunctival and episcleral tissue contributing to increased episcleral venous pressure and rise in intraocular pressure (IOP) [7, 8]. TTR amyloidosis has now several different systemic treatments that include not only liver transplant but also TTR stabilizing proteins (tafamidis), as well as gene therapy (RNA silencing—Onpattro, Tegsedi). All these reduce the systemic production of mutant TTR and significantly improves survival and quality of life of these patients, but do not affect the ocular TTR production and ocular complications. The mutant TTR can still be produced in the retinal and ciliary pigment epithelia [7, 9], contributing to a higher prevalence of glaucoma and a greater need for therapeutic intervention [10]. This continuous production of mutant TTR can explain the difficulty in reducing IOP and the course of glaucoma in TTR-FAP patients is accelerated and most of the time requires urgent surgical treatment [11]. Aqueous humour drainage devices have assumed an important role in several types of refractory glaucoma, either as a primary or secondary procedure [12].
TTR-FAP is an endemic disease on the northern coast of Portugal and patients are referred to Centro Hospitalar Universitário do Porto (CHUP), which is a referral centre for this disease, therefore accumulating a good casuistry. Currently, there are no published studies in the literature with such a large sample and long follow-up describing the outcomes of this surgical technique in the control of glaucoma in TTR-FAP patients. This study aimed to evaluate the surgical results of patients with glaucoma and TTR-FAP who underwent Ahmed glaucoma valve (AGV) implantation.
Materials and methods
Study design
A retrospective cohort study was performed, and it included patients with a diagnosis of TTR-FAP secondary glaucoma who underwent AGV implantation in the Ophthalmology Department of CHUP, between November 2010 and July 2019. This study was conducted following the tenets of the Declaration of Helsinki (1964). The authors ensured that the patients’ anonymity was carefully protected. Informed consent was signed for all procedures, following all the guidelines required by the institution with which all the authors are affiliated.
Participants
Indications for surgery included uncontrolled IOP despite maximum tolerated topical therapy with documented progression or previously failed glaucoma surgery. The inclusion criteria were as follows: glaucoma patients with a diagnosis of TTR-FAP confirmed by laboratory tests and collaborative ability to perform IOP evaluation with Goldmann’s applanation tonometry. Patients without 1 year of follow-up at the time of recorded review were excluded.
Parameters
The following variables were analysed:
-
demographic characteristics [gender, age, the timing of TTR-FAP laboratory diagnosis, first systemic symptoms and systemic treatment (liver transplant, pacemaker implantation and Tafamidis® therapy)];
-
previous ocular surgeries (vitrectomy, phacoemulsification and glaucoma surgeries);
-
type of surgery of AGV implantation [position of the tube—into anterior (AC) or posterior (PC) chamber—and isolated or combined with cataract surgery by phacoemulsification of crystalline];
-
preoperatively and postoperatively evaluation over time [best corrected visual acuity (BCVA), IOP, number of antihypertensive drugs, endothelial cell count (ECC) and optical disc nerve fiber layer thickness (OD-NFL)]; early (within 1 month) and late (>1 month) surgical complications; the need for further glaucoma surgery and follow-up time.
For numerical analysis, Snellen visual acuity was converted to logarithm of the minimum angle of resolution (logMAR) values. IOP was measured by Goldmann’s applanation tonometry. Combination medication eye drops were counted as two medications and oral carbonic anhydrase inhibitor was counted as one additional medication. Hypotony was defined as IOP ≤ 5 mmHg on two consecutive visits and hypertensive phase as the presence of an IOP ≥ 22 mmHg in the assessments of the first 3 months. OD-NFL thickness was measured by Structural Optical Coherence Tomography with the Spectralis® device (Heidelberg Engineering, Heidelberg, Germany). ECC count was measured with non-contact digital specular microscopy (KONAN® Noncon Robo-CA SP-8800).
Success was defined as an IOP ≥ 6 mmHg and ≤21 mmHg with no need for further glaucoma surgery and without loss of light perception at last follow-up: qualified success was defined as obtaining these results with medication use and complete success without medication use.
Surgical procedure
All surgical procedures were carried out by four experienced glaucoma surgeons. In all eyes, the FP7 model AGV (New World Medical®, Rancho Cucamonga, CA, USA) was used. Under subtenonian anaesthesia with lidocaine 2%, AGV was implanted according to the following technique: a limbal-based conjunctival incision is made to create a conjunctival flap between two recti muscles in the supero-temporal quadrant; cauterization of episcleral vessels; priming of the AGV by introducing balanced salt solution in the tube; placement of the plate 8–10 mm posterior to the corneal limbus and sclera fixation with 8–0 nylon; scleral flap creation (two-thirds thickness); performing AC paracentesis and injection of viscoelastic substance; cutting the tube to a bevel-shaped angle of 30o, creating a route 1–3 mm posteriorly to the corneoscleral limbus, parallel to the iris with a 23-gauge needle; inserting the tube in AC or PC; suture of the tube to the sclera with 8–0 nylon and recovering with an autologous scleral flap sutured with 10–0 nylon; finally the conjunctiva is closed with 10–0 nylon or 8–0 vicryl (by surgeon preference) and subconjunctival injection of cefazolin and dexamethasone are performed.
The tube was preferably placed in the PC if the patient was pseudophakic or had an indication of AGV implantation combined with cataract surgery by phacoemulsification of crystalline. The tube was placed in the AC if the patient was phakic, had a good ECC and depth of the AC.
In the postoperative period, all patients were treated with topical antibiotics for 8 days, oral and topical steroids at weaning, and non-steroidal anti-inflammatory drops for 6 months. Antiglaucoma medication was added when IOP increased above 21 mmHg or when progression was verified.
Statistical analysis
Statistical analysis was performed using the SPSS program (SPSS Statistics, version 22.0 for Windows, SPSS Inc., IBM, Somers, NY). The normality of the variables was evaluated by the Kolmogorov-Smirnov test. For preoperative and postoperative analysis, the Wilcoxon test and paired sample T test were used. The comparison between independent continuous variables was performed using the T Student test and Mann–Whitney test. The Fisher exact test was used for nominal scaled data. Kaplan–Meier survival analysis was used to determine the cumulative probability of surgical success, with failure of this treatment being defined as an IOP ≤ 5 mmHg and ≥22 mmHg, need of further glaucoma surgery, or loss of light perception at last follow-up. Univariate analysis using the log-rank test compared Kaplan–Meier survival curves. Spearman’s bivariate correlation test was applied to study linear correlations. The p < 0.05 were considered statistically significant.
Results
Demographic data
This study included 114 eyes of 87 patients with TTR-FAP secondary glaucoma who underwent AGV implantation. The mean age was 51.26 ± 6.87 years (y) [range, 39–68] at the time of AGV implantation.
The mean age at onset of TTR-FAP systemic symptoms was 33.47 ± 9.79 y [range, 18–65], and the mean age at diagnosis was 30.84 ± 10.78 y [range, 6–67]. 95.6% of cases underwent liver transplantation at mean age of 37.31 ± 7.95 y [range, 24–57], on average 5.16 ± 3.75 y after first TTR-FAP systemic symptoms and 90.8% underwent pacemaker implantation at mean age of 39.89 ± 8.37 y [range, 26–70], on average 7.14 ± 5.92 y after first TTR-FAP systemic symptoms. Only 6.3% of our sample was treated with tafamidis meglumine started at mean age of 56.14 ± 11.01 y [range, 39–67], on average 3.40 ± 2.30 y after first TTR-FAP systemic symptoms. (Table 1)
Previous ocular surgeries
Thirteen eyes (11.40%) had glaucoma surgeries before AGV implantation, trabeculectomy in 9 eyes, cyclophotocoagulation in 1 eye, EX-PRESS® implant in 1 eye, trabeculectomy and cyclophotocoagulation in 1 eye, and trabeculectomy, nonpenetrating deep sclerectomy, and cyclophotocoagulation in 1 eye. 56 eyes (49.10%) underwent vitrectomy on average 2.43 ± 1.80 y [range, 0.21–6.92] before AGV implantation and 17.11 ± 5.78 y after first TTR-FAP systemic symptoms. Cataract surgery by phacoemulsification of crystalline was performed previously in 31 eyes (27.19%): 11 combined with vitrectomy, 4 before vitrectomy, 12 after vitrectomy (mean of 2.55 ± 1.75 y after vitrectomy), and 4 without previous vitrectomy. The mean time between first TTR-FAP systemic symptoms and cataract surgery was 17.62 ± 6.95 y.
Type of surgery of AGV implantation
78 eyes (68.42%) underwent AGV implantation alone (64 eyes with the tube in AC and 14 in PC). 36 eyes (31.58%) underwent AGV implantation combined with cataract surgery by phacoemulsification of crystalline (20 eyes with the tube in AC and 16 in PC).
Outcomes
IOP
Mean follow-up duration was 3.81 ± 2.11 y [range, 1.00–8.28 y]. Preoperative BCVA increased from 0.31 ± 0.31 logMAR to 0.25 ± 0.30 logMAR at the last visit (p = 0.122). This improvement was significant (p = 0.016) in eyes who underwent AGV implantation combined with cataract surgery by phacoemulsification of crystalline with preoperative BCVA of 0.43 ± 0.34 logMAR and final BCVA of 0.28 ± 0.36 logMAR.
Comparing to preoperative values, the mean IOP was reduced from 28.20 ± 7.01 mmHg to 8.61 ± 6.64 mmHg on 1st day. There was a slight increase to 9.64 ± 5.75 mmHg at 1st week, 13.69 ± 7.02 mmHg at 2nd week, and a maximal mean value of 17.36 ± 6.46 mmHg at 1st month. Mean IOP decreased to 16.67 ± 5.43 mmHg at 2nd month, 16.57 ± 3.92 mmHg at 3rd month, 15.40 ± 4.43 mmHg at 6th month, 13.68 ± 3.74 mmHg at 1st year and 12.87 ± 3.76 mmHg at the final visit (Fig. 1). Mean postoperative IOPs were all significantly reduced compared to baseline (p < 0.001). The mean number of antihypertensive ocular medications decreased from 3.89 ± 0.66 to 1.86 ± 1.43 at the final visit (p < 0.001).
The ocular hypertensive phase was observed in 50.0% of eyes, on average at 39.61 ± 23.19 days after AGV implantation. Although not statistically significant, preoperative IOP was higher in eyes with hypertensive phase (p = 0.075). Type of AGV surgery (isolated vs combined with cataract surgery by phacoemulsification of crystalline) (p = 0.546), previous vitrectomy (p = 0.574), previous cataract surgery (p = 1.000), and early postoperative complications (p = 0.461) seem not to influence the presence of hypertensive phase.
ECC and OD-NFL
Mean ECC decreased from 2548.36 ± 564.16cel/mm2 to 2246.41 ± 697.889 cel/mm2 at 1 y (p = 0.006) and 2031.02 ± 542.42cel/mm2 at the last visit (p < 0.001). There wasn´t a statistically significant difference (p = 0.253) in the mean percentage of ECC loss per year at the 1st year after AGV implantation (7.73%/year) comparing to at the last visit (5.36%/year). There wasn´t a statistically significant difference in the mean percentage of ECC loss per year (p = 0.129) and mean ECC at the last visit (p = 0.831) between a position of the tube into AC and PC. Although not statistically significant, mean ECC loss per year at the last visit was higher in eyes that underwent AGV implantation combined with cataract surgery (p = 0.279), with early (p = 0.615) or late postoperative complications (p = 0.228).
Mean OD-NFL thickness was decreased from 82.09 ± 18.46 to 68.54 ± 20.72 at 6th months (p < 0.001). Mean OD-NFL thickness was 64.09 ± 21.37 at 1 year and it was similar (p = 0.928) to mean OD-NFL thickness at the last visit, 63.85 ± 23.32, showing potential structural stability after 1st year of follow-up.
Postoperative complications
Prevalence of early postoperative complications was 17.09% (20 eyes). (Table 2) The most common was hypotony with or without atalamia that occurred in 9 eyes (7.89%). Other early complications were as follows: mild hyphema in 3 eyes (2.63%), vitreous haemorrhage in 3 (2.63%), tube obstruction in 2 (1.75%), corneal ulcer in 2 (1.75%) (one of which with simultaneously hypotony already accounted above), transient choroidal detachment in 1 (0.88%) and suprachoroidal haemorrhage occurred in 1 (0.88%). At the last visit, eyes with early postoperative complications had a similar mean of BCVA (p = 0.718), IOP (p = 0.201), number of glaucoma medications (p = 0.707), ECC (p = 0.682), OD-NFL (p = 0.663), and surgical success rate (p = 0.125) than eyes without.
Prevalence of late postoperative complications was 7.89% (9 eyes). Corneal decompensation was the most common, occurred in 4 eyes (3.51%), one at 1 year, two at 3 years and the other at 7 years after AGV implantation. In all eyes, the tube was replaced to the PC and one of them needed descemet stripping endothelial keratoplasty after 1 year. Tube exposure occurred in 2 eyes (1.75%), intraocular lens subluxation in 2 (1.75%) and bleb encapsulation in 1 (0.88%). At the last visit, eyes with late postoperative complications had similar mean of BCVA (p = 0.967), IOP (p = 0.102), number of glaucoma medications (p = 0.584), OD-NFL (p = 0.309), surgical success rate (p = 0.080), but lower ECC (p = 0.020) than others.
Additional surgeries
Fourteen eyes (12.28%) required additional glaucoma surgery and these underwent cyclophotocoagulation at 2.00 ± 1.26 y after AGV implantation. These had a lower (p = 0.005) number of glaucoma medications (μ = 0.86 ± 1.29) than eyes without additional glaucoma surgery (μ = 2.00 ± 1.39) at the last visit. Thirty-five eyes (30.70%) needed vitrectomy at 2.25 ± 1.84 y after AGV implantation due to vitreous amyloid and had lower (p = 0.050) BCVA (μ = 0.33 ± 0.33) than eyes without (μ = 0.21 ± 0.27) at the last visit. Twenty eyes (17.54%) underwent cataract surgery at 2.70 ± 2.11 y after AGV implantation and had a lower (p = 0.003) ECC (μ = 1672.00 ± 556.78) at the last visit than others (μ = 2109.71 ± 510.00). In this subgroup surgical success rate (75%) was lower than those who didn´t undergo cataract surgery after AGV (90.42%), even though there was no statistically significant difference (p = 0.069). Clinical retinal angiopathy (defined as the presence of some of the following changes on the ocular fundus: cotton wool spots, retinal haemorrhages, peripheral neovascularization, tortuous retinal vessels, retinal vein occlusion, macular oedema, retinal vascular sheathing or pinpoint white amyloid deposits over the retinal surface) was observed in 22.8% of eyes in the last visit and was associated with lower BCVA (p = 0.120).
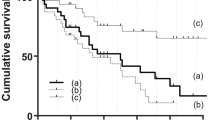
Surgical success
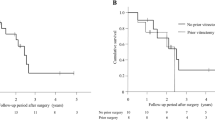
Surgical success was observed in 100 eyes (87.72%), qualified in 78 (68.42%) and complete success in 22 eyes (19.30%), at a mean of 3.81 ± 2.11 years, corresponding to the final follow-up. Kaplan–Meier analysis indicated probabilities of success of 0.98 at 1 year, 0.97 at 2 years, 0.95 at 3 years, 0.89 at 4 years, 0.77 at 5 years and 0.72 at 6 years (Fig. 2). Success rates were similar between eyes that underwent AGV implantation alone or combined with cataract surgery by phacoemulsification of crystalline (p = 0.810), with or without previous glaucoma surgery (p = 0.938), previous vitrectomy (p = 0.173), or previous cataract surgery by phacoemulsification of crystalline (p = 0.558).
AGV implantation alone or combined with cataract surgery by phacoemulsification of crystalline (A), with or without previous glaucoma surgery (B), with or without previous vitrectomy (C) and with or without previous cataract surgery by phacoemulsification of crystalline (D). The success rate for eyes with Familial Amyloid Polyneuropathy (TTR-FAP) glaucoma who had undergone AGV implantation was decreased with time. Percent of success cases and the number of eyes during the follow-up period are shown in the box. The median survival time was 8.00 years. The success rates were not significantly different between the eyes of each group. p-value in (A) = 0.810, p-value (B) = 0.938, p-value (C) = 0.173 and p-value (D) = 0.558. p-value was calculated using log-rank analysis.
There was a linear correlation between age at pacemaker procedure and AGV implantation success (r = 0.281, p = 0.006). Although not statistically significant, there were other positive correlations between some characteristics of TTR-FAP patients and AGV implantation success (Table 3).
Discussion
There are only a few reports in the literature on types of glaucoma procedures in TTR-FAP patients. Initially, Kimura et al. [7] demonstrated that trabeculectomy with mitomycin C (MMC) appeared to be the most promising treatment based on their previous short-term results. However, Kawaji et al. [13] provided a longer follow-up of the previous study and revealed that trabeculectomy with MMC seemed to be limited to TTR-FAP glaucoma and to have significant complications. Accordingly, they emphasized the need for studies with glaucoma drainage implants. Although we have no published data on the effects of trabeculectomy on TTR-FAP glaucoma, our experience until 2009 showed us that this was not a good option for these patients, as the success rate was very low. For this reason, from 2010, we decided to change the approach to aqueous humour drainage devices. We chose the Ahmed glaucoma valve implant because it was the device we had experience within refractory glaucomas. Recently, Kakhara et al. [14] suggested that Baerveldt glaucoma drainage implant surgery may currently be the optimal treatment for TTR-FAP secondary glaucoma showing good outcomes in 5 eyes of 4 patients.
Our previous studies [15, 16] demonstrated the short-term outcomes of AGV implantation in patients with TTR-FAP secondary glaucoma, and suggested that AGV implantation may be the most promising treatment. Our present study provided good long-term follow-up results. Comparing to Kawaji et al. [13], we report better cumulative probability of treatment success with 0.98 at 1 year (vs 0.76), 0.97 at 2 years (vs 0.67), 0.95 years at 3 years (vs 0.53) and 0.89 at 4 years (vs 0.11). At 6 years of follow-up, we had 0.72 of the cumulative probability of treatment success. We have only 9 eyes with 7 or more years of follow-up so statistically relevant conclusions cannot be drawn. In addition, surgery for further IOP control was required in 12.28% of eyes and these with only one procedure (cyclophotocoagulation), much lower than 57% with one and 33% with more than one additional treatment, reported by Kawaji et al. [13], and lower than 20% reported by Kakihara [14]. The interval to additional glaucoma surgery was similar, of around 2 years. Latasiewicz et al. [11] reported good results of nonpenetrating deep sclerectomy performed in 4 eyes with TTR-FAP secondary glaucoma. However, they had a short follow-up (7, 20, 23 and 38 months) and the only eye with 38 months follow-up needed laser goniopuncture at 30 months after surgery.
In our study, IOP postoperative was higher in 1–2 months following the operation that is correlated with the ‘hypertensive phase’ present in 50.0% of eyes and commonly described following AVG implantation (ranging from 40–80%) [17].
Concerning complications, the prevalence of early and late postoperative complications was 17.09% and 7.89%, respectively, without significant change of success rate (p > 0.05). Hypotony was the most common early complication and occurred in 9 eyes (7.89%). A ‘hypotensive’ phase was recorded in 13 and 15% of patients in the AVB and ABC studies, respectively [18, 19]. Corneal decompensation was the most common late complication, occurred in 4 eyes (3.51%), in these the tube was replaced to the PC and one of them needed descemet stripping endothelial keratoplasty after 1 year. In literature, corneal decompensation has been reported to be 9–27% in the long term [20,21,22].
Regarding systemic disease, we observed a statistically significant positive correlation between age at pacemaker procedure and AGV implantation success. Although not statistically significant, there were positive correlations between some other characteristics of TTR-FAP patients (age at 1st symptoms of TTR-FAP, age at 1st glaucoma surgery, age at vitrectomy before AGV implantation and age at AGV implantation) and AGV implantation success. There is greater overall AGV implantation success when the need to carry out this type of intervention occurs at older age. This may be explained by less severe phenotypes of the disease since these patients needed treatments later.
This is the first study to document the long-term effects of drainage implants on TTR-FAP associated glaucoma. Two of the strengths of this study are the large sample size and long follow-up period, which allows a better assessment of the outcome. The major limitation is its retrospective design. Further limitations are surgical procedures performed by four different surgeons and the absence of a control group to compare with other surgical techniques. We performed only AGV for TTR-FAP glaucoma because it is the only aqueous humour drainage device that we have surgical experience. However, we think that the Baerveldt glaucoma implant, like other devices, may have a great chance of having good results as well. Studies with other devices are needed for this population.
Although there are no comparative studies between filtering surgery and drainage devices in this pathology, our experience suggests that the AGV can be superior to filtering surgery, possibly because these patients had a modified and fragile conjunctiva, associated with amyloid deposition, previous surgeries (namely vitrectomy) and the cumulative use of multiple eye drops (not only for glaucoma but also for dry eye) [15].
Overall, our study provides long-term follow-up showing that the AGV implantation is a safe and effective option in TTR-FAP secondary glaucoma, with excellent success and low complication rates.
Summary
What was known before
-
The course of glaucoma in transthyretin-related familial amyloid polyneuropathy patients is accelerated, most of the time requires urgent surgical treatment and it is difficult to control.
What this study adds
-
Ahmed glaucoma valve implantation is a safe and effective option in transthyretin-related familial amyloid polyneuropathy secondary glaucoma, with excellent success and low complications rates.
References
Beirão M, Matos E, Reis R, Beirão I, Costa PP, Torres P. Spatial visual contrast sensitivity in liver transplanted Portuguese familial amyloidotic polyneuropathy (ATTR V30M) patients. Amyloid .2012;19:152–5.
Planté-Bordeneuve V, Kerschen P. Transthyretin familial amyloid polyneuropathy. Handb Clin Neurol. 2013;115:643–58.
Beirão JM, Moreira LM, Oliveira JC, Menéres MJ, Pessoa BB, Matos ME, et al. Aqueous humor erythropoietin levels in open-angle glaucoma patients with and without TTR V30M familial amyloid polyneuropathy. Mol Vis. 2014;20:970–6.
Rosa AM, Quadrado MJ, Ferrão J, Marques I, Pereira H, Costa E, et al. Manifestações Oculares de Polineuropatia Amiloidótica Familiar Tipo I em doentes submetidos a transplante hepático. Oftalmologia .2009;33:177–83.
Beirão M, Matos E, Beirão I, Pinho-Costa P, Torres P. No ocular involvement in familial amyloidotic polyneuropathy ATTR V30M domino liver recipientes. Transpl Int. 2012;25:646–51.
Conceição I, Carvalho M. Clinical variability in type I familial amyloid polyneuropathy (Val30Met): comparison between late- and early-onset cases in Portugal. Muscle Nerve. 2007;35:116–8.
Kimura A, Ando E, Fukushima M, Koga T, Hirata A, Arimura K, et al. Secondary glaucoma in patients with familial amyloidotic polyneuropathy. Arch Ophthalmol. 2003;121:351–6.
Beirão NM, Matos ME, Meneres MJ, Beirão IM, Costa PP, Torres PA. Vitreous surgery impact in glaucoma development in liver transplanted familial amyloidosis ATTR V30M Portuguese patients. Amyloid .2012;19:146–51.
Kawaji T, Ando Y, Nakamura M, Yamamoto K, Ando E, Takano A. Transthyretin synthesis in rabbit ciliary pigment epithelium. Exp Eye Res. 2005;81(Sep):306–12.
Martins AC, Rosa AM, Costa E, Tavares C, Quadrado MJ, Murta JN. Ocular manifestations and therapeutic options in patients with familial amyloid polyneuropathy: a systematic review. Biomed Res Int. 2015;2015:282405.
Latasiewicz M, Millá E, Giralt J, Molina JJ, Matas J. Nonpenetrating deep sclerectomy as an effective treatment of glaucoma related to familial amyloid polyneuropathy. J Glaucoma. 2015;24:e80–3.
Lee CK, Ma KT, Hong YJ, Kim CY. Long-term clinical outcomes of Ahmed valve implantation in patients with refractory glaucoma. PLoS One. 2017;12:e0187533.
Kawaji T, Inoue T, Hara R, Eiki D, Ando Y, Tanihara H. Long-term outcomes and complications of trabeculectomy for secondary glaucoma in patients with familial amyloidotic polyneuropathy. PLoS One. 2014;9:e96324.
Kakihara S, Hirano T, Imai A, Miyahara T, Murata T. Baerveldt glaucoma drainage implant surgery for secondary glaucoma in patients with transthyretin-related familial amyloid polyneuropathy. Jpn J Ophthalmol. 2020;64(Sep):533–8.
Borges T, Figueiredo A, Reis R, Sampaio I, Menéres MJ. Válvulas de Ahmed em doentes com Polineuropatia Amiloidótica Familiar. Oftalmologia .2015;39:231–7.
Figueiredo A, Vale C, Casal I, Sousa P, Sampaio I, Menéres MJ. Válvulas de Ahmed na cirurgia de glaucoma: a nossa experiência. Oftalmologia .2014;38:149–56.
Ayyala RS, Zurakowski D, Monshizadeh R, Hong CH, Richards D, Layden WE, et al. Comparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucoma. Ophthalmic Surg Lasers. 2002;33:94–101.
Christakis PG, Tsai JC, Kalenak JW, Zurakowski D, Cantor LB, Kammer JA, et al. The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology .2013;120:2232–40.
Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, et al. Ahmed Baerveldt Comparison Study Group. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology .2011;118:443–52.
Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136:464–70.
Kook MS, Yoon J, Kim J, Lee MS. Clinical results of Ahmed glaucoma valve implantation in refractory glaucoma with adjunctive mitomycin C. Ophthalmic Surg Lasers. 2000;31:100–6.
Riva I, Roberti G, Oddone F, Konstas AG, Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357–67.
Author information
Authors and Affiliations
Contributions
AM and RV were responsible for the acquisition, analysis, or interpretation of data for the work; drafting the work; the final approval of the version to be published; and the agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AF, RR, IS, JMB and MJM were responsible for the conception or design of the work; revising it critically for important intellectual content; final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PRECIS: Our study provides long-term follow-up showing that the Ahmed glaucoma valve implantation is a safe and effective option in hereditary transthyretin amyloidosis secondary glaucoma, with excellent success and low complication rates.
Rights and permissions
About this article
Cite this article
Marta, A., Vieira, R., Figueiredo, A. et al. Ahmed valve for secondary glaucoma in patients with hereditary transthyretin amyloidosis. Eye 36, 111–118 (2022). https://doi.org/10.1038/s41433-021-01443-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01443-y
This article is cited by
-
Long-term surgical results of trabeculectomy for secondary glaucoma in Val30Met hereditary transthyretin amyloidosis
Scientific Reports (2023)
-
Microhook ab interno trabeculotomy for secondary glaucoma in patients with hereditary transthyretin amyloidosis
Japanese Journal of Ophthalmology (2023)
-
Suture trabeculotomy ab interno for secondary glaucoma in Japanese patients with Val30Met hereditary transthyretin amyloidosis
Scientific Reports (2022)