Abstract
Purpose
To evaluate the impact of blood sugar level on ocular measures, including refractive error (RE), amplitude of accommodation (AoA), and lag of accommodation (LoA), in pre-presbyopes with type-1 diabetes.
Method
The fasting blood sugar (FBS) and ocular measures of type-1 diabetes patients (age: 14–39 years; n = 30) on insulin treatment was recorded while they fasted on two separate visits, at baseline and 3 months later. The AoA and LoA was measured with the appropriate spectacle correction worn. The Welch’s t-test was used for comparison of the baseline measures between the normal FBS ≤ 7 (n = 10) and higher FBS > 7 (n = 20) patients, and the paired t-test used to investigate for differences between the baseline and follow-up data in patients with changes in FBS.
Results
On average, the spectacle correction for the normal FBS group was marginally more myopic (RE: −0.30 ± 0.67 D vs. +0.18 ± 1.00 D, p = 0.032), and they showed greater AoA (5.38 ± 1.08 D vs. 3.68 ± 1.43 D, p < 0.001) and lower LoA (1.00 ± 0.30 D vs. 1.30 ± 0.38 D, p = 0.004) compared with the higher FBS group at baseline. On the follow-up visit attended by 25 patients, the FBS of 15 patients was reduced by an average of 7.0 mmol/L, 8 patients had an average increase of 5.2 mmol/L, while 2 patients recorded no changes relative to the baseline. The patients whose FBS was reduced showed improvement in the mean AoA from 3.78 ± 1.58 D to 4.88 ± 1.61 D (p < 0.001) and a reduction in the mean LoA from 1.37 ± 0.40D to 0.87 ± 0.19D (p < 0.001), whereas those with deteriorated control of the FBS showed an opposite trend.
Conclusions
Controlling hyperglycemia improves ocular accommodation in type-1 diabetes.
Similar content being viewed by others
Introduction
Diabetes is characterized by a sustained higher blood glucose concentration accompanied by the classic trio of symptoms: polydipsia, polyphagia, and polyuria [1]. Thus, the blood glucose level is crucial in the pathogenesis of diabetic complications, and as such treatments for diabetes are targeted at lowering the blood glucose concentration [2]. Regular measurement or monitoring of the blood glucose concentration is, therefore, a routine practice in the management of a patient with diabetes. While several forms of diabetes may be recognized based on the etiology, most patients are diagnosed with type-1 or type-2 diabetes according to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [1].
Diabetes is implicated in a myriad of both anterior and the posterior segments eye disorders of which the commonly studied include diabetic retinopathy, diabetic cataract, refractive error (RE), optic neuropathy, glaucoma, and ocular surface disorders. Diabetics often experience blurred vision, a symptom largely attributed to changes in the refractive system of the eye. Wiemer et al. found that complaints of blurred vision during hyperglycemia may not be limited to changes in the refractive properties [3]. The ocular accommodative system, which plays an important role in the eye’s ability to fixate and maintain visual clarity at varying distances [4], can be impaired in hyperglycemia. Reports by Braun et al. [5] and Khan et al. [6] suggest that the eyes’ accommodative ability may be affected much earlier in diabetic subjects than their age-matched healthy subjects. These observations allude to the notion that the visual problems encountered in type-1 diabetes may be related to the impairment of the accommodative system of the eye.
Several confounding factors are involved in determining the accommodative status in diabetes, some of which include age, blood glucose level, duration of diabetes, and high blood pressure [5,6,7]. We recently reported that the changes in blood glucose level alone significantly influenced the eye’s amplitude of accommodation (AoA) and response to a stimulus, after the control of other determining factors [7]. There is, however, no compelling data demonstrating the potential benefit of adequate control of type-1 diabetes with insulin treatment on the ocular accommodative status of pre-presbyopes. Also, in the case of the refractive changes in diabetes, different studies have reported conflicting outcomes. The contradictions may result from racial differences in the distribution of RE and binocular and accommodative characteristics [8, 9]. The purpose of our current study was to assess whether these ocular changes in RE and accommodation were associated with the blood sugar level and to determine the impact of lowering of the blood sugar on these ocular measures in a diabetic patient of African descent.
Methods
Ethical approval and subject recruitment
Approval for this study was obtained from the Institutional Review Board of the University of Cape Coast, Ghana (UCCIRB/CHAS/2015/087). Also, permission was obtained from the head of the Diabetes Clinic, Cape Coast Teaching Hospital, Ghana. The study protocol followed the guidelines provided in the declaration of Helsinki for the use of human subjects in research. Adults gave written consent to participate and the minors provided verbal assent in addition to the consent from their parents.
Thirty patients diagnosed with type-1 diabetes and receiving treatment at the Cape Coast Teaching Hospital, between October 2015 and May 2016 were prospectively enrolled. A minimum sample size of 22 was calculated based on the requirement to determine the difference between two dependent means (matched pairs), using a significance level of 5% (p = 0.05), power of study of 80%, and effect size of 0.8 in the G*Power software. A definitive diagnosis of type-1 diabetes was made by a diabetes physician specialist based upon clinical signs as proposed by Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, plus the presence of fasting plasma C-peptide concentration <0.2 nmol/l [1]. The type-1 diabetes management included injecting rapid‐acting insulin about 30 min before a meal, in line with recent recommendations [10]. Eligibility criteria for the study included established diagnosis of type-1 diabetes, being 39 years old or younger with the near vision of N8 (nonpresbyopic), having a corrected distance visual acuity of 6/9 or better in both eyes, the absence of ocular disease, and not prescribed any medication known to affect accommodation.
Study protocol
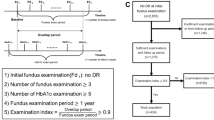
The 30 participants, who were type-1 diabetes patients on insulin treatment, underwent an overnight fast lasting for 10–12 h to prepare them for measurement of the fasting blood glucose (FBS) at baseline. For the follow-up visit scheduled 3 months later, patients continued with the insulin without changes in the treatment regimen. Again, patients fasted for the same length of time before re-examination on the follow-up visit to ensure consistency. On each visit, the FBS was measured first, followed by the RE to determine the current glasses correction to be worn before the recording of the AoA and lag of accommodation (LoA).
The examination period lasted for an hour (starting from 8 a.m. to 9 a.m.), and the FBS was first recorded followed by the ocular examination on each visit. To ensure a consistent time from the last insulin dose on both visits, participants were instructed to take their last meal between 7 and 8 p.m., and the insulin injection was always 30 min before meals. The examiner recording the ocular measures on subjects was blinded to their FBS results until the study was completed. Based on the baseline FBS, participants were then categorized as having either normal FBS (≤7 mmol/l) or higher FBS (>7 mmol/l) for comparison of the RE, AoA, and LoA findings.
On the 3-month follow-up visit, only patients with either a reduction or an increase in the FBS were selected for repeated measurement of RE, AoA, and LoA. Taking into consideration a 15% test–retest variability in the FBS readings [11], a difference of ≥1.11 mmol/L (20 mg/dl) between the baseline and follow-up FBS values was considered as a clinically significant change. Based on this criterion, 15 subjects had a reduction in the FBS while 8 showed an increase in the FBS. Two patients had no significant changes in FBS and 5 patients were lost to follow-up. Thus, only 23 patients underwent repeated measurements of the RE, AoA, and LoA for the follow-up study.
Ocular measurements
The distance and near visual acuities were measured using the Snellen visual acuity chart monocularly for each participant. Anterior and posterior segments examinations were performed to rule out the presence of any ocular disease. Static retinoscopy was then performed followed by subjective refraction by an optometrist (blinded to the FBS group of the patient) to estimate the RE in both eyes. The final distance correction was determined subjectively, using the maximum plus lens that provided a distance VA of 6/9 or better. All participants wore a trial frame with their distance correction in place before proceeding with the measurement of AoA and LoA. Each measurement was repeated three times and the two closest readings averaged and recorded.
Modified dynamic retinoscopy (MDR)
To measure the objective AoA, the MDR procedure previously described by Rutstein et al. was used [12]. This technique employs the traditional “push-up” of a target to blur approach, although the practitioner objectively determines the near point of accommodation of subject monocularly, using a retinoscopy reflex. At the starting point with the target at 40 cm (i.e., the plane of retinoscope) from the fixating subject, the practitioner sees a bright, full, and fast “with” reflex. This is because, technically, the accommodative response by the eye is usually lower than the accommodative stimulus/demand. So, therefore, the starting bright, full, and fast “with” reflex, is the endpoint for accommodative response at 40 cm. And as the target and retinoscope are pushed closer and the subject keeps fixating, the accommodative response increases. As a result, the bright, full, and fast “with” reflex remains until when the near point of accommodation is reached. At that point, the examiner observes a persistently narrow, slow, and dull reflex.
Briefly, with a patient wearing his/her distance refractive correction and one eye occluded, the patient was instructed to clearly focus with the fellow eye and read aloud letters on a near card attached to the front of a streak retinoscope (Welch Allyn) held by an optometrist at 40 cm. The N8 line of letters was used as the accommodative target since it was the letter size that remained readable under the test condition (i.e., dim light and with the streak of light from the retinoscope incident on that eye). The optometrist scoped the eye with a vertical streak of retinoscope to observe the reflex. Upon observing a bright, full, and fast “with” reflex in the eye, using the retinoscope, the target attached to the retinoscope was moved closer to the patient until the reflex persistently appeared slower, narrower, and dull. The AoA (diopters) was quantified as the reciprocal of the distance from the patient’s eye (in meters) to the position of the retinoscope where there was a persistent change in the reflex. The same procedure was repeated on the other eye and recorded.
Monocular estimation method (MEM)
The MEM, an objective method, was employed to measure the LoA of the right eye first and then the left eye. Briefly, the patient was seated at 40 cm from an optometrist for this procedure. An accommodative target (N8) was set in place and the patient fixated on the accommodative target. Trial lenses were inserted into the trial frame while scoping the eye for the neutrality of the accommodative reflex. To prevent a patient from accommodating over the correcting lens, the examiner kept each lens in place for about a second during neutralization, starting with the +1.00 D lens and using the bracketing technique to reduce the number of lens changes. The trial lens power used to achieve neutrality of the accommodative reflex was recorded as the LoA. The test was done over the subjective distance correction already in place.
Fasting plasma glucose measurement
Subjects underwent a 10–12 h fast and avoided any strenuous physical activity prior to the taking of venous blood samples. In addition, subjects were not to smoke or take in any alcohol within the period of fast. Blood samples were taken from subjects before 10 a.m., fluoridated on ice and centrifuged to collect the fluoride plasma, and kept frozen at −80°C until ready for biochemical analysis (Glucose 201+ Analyzer, Albumin Systems; HemoCue, Sweden).
Intra-rater reliability
Before the study, we determined the intra-rater reliability for RE, AoA, and LoA by the intraclass correlation coefficient and 95% limits of agreement, based on data obtained from ten healthy subjects examined on two separate occasions by the optometrist. All three retinoscopy-based measurements by the examiner showed excellent reliability and reproducibility (RE: 0.92, ±0.30 D; AoA: 0.90, ±0.38 D, LoA: 0.96, ±0.18 D) comparable to previous studies [13,14,15].
Data analysis
The analysis was performed using the GraphPad Prism software, version 21. Data were presented as mean (±SD) and included results from both eyes of subjects. The one-sample Kolmogorov–Smirnov test was used to test for deviations from a normal distribution. Welch’s t-test was used to determine differences between the higher FBS group and the lower FBS group. The paired t-test was used to determine the statistical difference between measurements taken at baseline and during a follow-up visit. The level of significance was set at p value < 0.05.
Results
Baseline characteristics of diabetics in the cross-sectional study
At the baseline, 10 subjects (2 males and 8 females) recorded normal FBS (i.e., ≤ 7 mmol/L), whereas 20 patients (6 males and 14 females) had higher FBS (i.e., >7 mmol/L). The participants age was normally distributed (D (39) = 0.1232; p = 0.707) with a mean of 27.7 ± 7.8 (age range: 14–39 years) (Fig. 1). There was no difference between the mean age of the normal FBS group (28.5 ± 8.2 years) compared with the higher FBS group (27.3 ± 7.5 years, p = 0.586, Table 1). The mean duration of diabetes was also similar for the two groups (normal FBS: 4.3 ± 3.4 years; higher FBS: 5.1 ± 4.2 years, p = 0.432). Other visual and general health information, including the RE, weight, and blood pressure, for each group are presented in Table 1.
Associations between FBS and RE, AoA, LoA at baseline
The mean spherical equivalent RE was marginally myopic in the normal FBS group compared with the higher FBS group (−0.30 ± 0.67 D vs. +0.18 ± 1.0 D, respectively; p = 0.032). It was observed that the diabetic subjects with higher baseline FBS showed lower AoA (3.68 ± 1.43 D vs. 5.38 ± 1.08 D, p = 0.004) and higher LoA (1.30 ± 0.38 D vs. 1.00 ± 0.30 D, p = 0.003) compared with their counterparts with lower FBS (Table 1).
Follow-up study on the impact of FBS on RE, AoA, and LoA
With continuous insulin treatment for 3 additional months from the baseline, a total of 23 type-1 diabetes patients showed differences between the follow-up FBS and the baseline FBS of at least 1.1 mmol/L or above. The 23 patients were then assigned into two groups based on the direction of change of the follow-up FBS—the reduced FBS group and the group with deteriorated control of the FBS. The reduced FBS group (n = 15) showed control of diabetes as their mean baseline FBS of 15.1 ± 7.2 mmol/L dropped to 8.1 ± 4.1 mmol/L on the follow-up visit. The FBS reduction was accompanied by a significant improvement in the mean AoA from 3.78 ± 1.58 D to 4.88 ± 1.61 D, p < 0.001, and a reduction in the mean LoA from 1.37 ± 0.40 D to 0.87 ± 0.19 D, p < 0.001 (Fig. 2a). We observed, however, that there was no significant (p = 0.423) change in mean RE following the reduction in FBS (baseline RE: + 0.35 ± 1.13 D vs. follow-up RE: + 0.27 ± 0.97 D; Fig. 2a).
Comparisons of the amplitude of accommodation (AoA), lag of accommodation (LoA), and refractive error (RE) at baseline and following (a) a reduction or (b) an increase of the fasting blood sugar (FBS) in type-1 diabetic patients. Paired t-test was used to determine the statistical difference between measurements taken at baseline and during the follow-up visit.
Conversely, the group with deteriorated control of FBS (n = 8) recorded a mean baseline FBS of 7.8 ± 3.9 mmol/L that increased to 13.0 ± 5.7 mmol/L on the follow-up visit. The FBS increase was accompanied by a reduction in the mean AoA (4.95 ± 1.58 D vs. 3.39 ± 1.66 D, p = 0.010) and an increase in the mean LoA (0.88 ± 0.39 D vs. 1.28 ± 0.41 D, p < 0.001). Also, however, the increase in FBS did not affect the RE (−0.53 ± 0.87 D vs. −0.53 ± 0.81 D, p = 0.999; Fig. 2b).
Discussion
Diabetes has several ocular complications, the commonly known ones include diabetic retinopathy, cataract, optic neuropathy, glaucoma, ocular surface disorders, and fluctuations in RE [16]. There is evidence suggesting that adequate control of blood glucose can delay, prevent, or reverse some of these ocular complications [17, 18]. Among these ocular disorders, fluctuations in RE are one of the most studied causes of vision disturbance in type-1 diabetic patients. Reports from different studies regarding the association between blood sugar level and refractive changes remain controversial. Duke first explored the relationship between blood glucose and RE [19]. He concluded that hyperglycemia was associated with a myopic shift, whereas hypoglycemia was associated with a hyperopic shift. While this assertion has been confirmed by Lin et al. [20], Okamoto et al. [21], and Sonmenz et al. [22], other researchers [3, 23] have equally refuted it. Interestingly, data from some of the studies that argued in favor of hyperopic shift during intensive glycaemic control (i.e., hypoglycemia) show that some of their study participants experienced a myopic shift instead [22]. For instance, Sonmenz et al. demonstrated that intensive glycaemic control, evident by a mean reduction of the blood glucose level from 19.76 to 7.42 mmol/L, led to a hyperopic shift on average [22]. On an individual basis, however, only half of their eighteen study participants became hyperopic, while the remaining became more myopic or showed no refractive changes after treatment [22]. Likewise, our follow-up study did not find any refractive changes to accompany the blood glucose changes on average in the diabetes patients, but some of the patients recorded either myopic and hyperopic changes. Based on this information, we hypothesize that diabetic patients may respond differently in terms of the refractive changes that accompany changes in the blood sugar level. The key finding of our study is that the control of hyperglycemia in type-1 diabetic patients improves the eye’s accommodative ability on the average notwithstanding the absence of refractive changes.
Our study, conducted in type-1 diabetes subjects of African descent, showed that type-1 diabetes patients with normal blood glucose were marginally more myopic compared with those with higher blood glucose control. However, the follow-up investigation in the reduced FBS group, whose mean FBS reduced from 15.1 ± 7.2 to 8.1 ± 4.1 mmol/L, and the group with deteriorated diabetes control having an increase in the mean FBS from 7.8 ± 3.9 to 13.0 ± 5.7 mmol/L, showed no significant refractive changes (i.e., no myopic shift or hyperopic shift). Thus, our findings could not support the previous studies which associated a myopic or hyperopic shift with the changes in blood sugar by diabetes patients. Wiemer and his colleagues investigated the differences in refraction and optical aberrations in 25 diabetic patients when they experienced hyperglycemia (blood glucose >10 mmol/l) and blurred vision and when both conditions were absent [3]. In their study which observed a mean reduction of 5.9 mmol/l in the blood sugar on the follow-up visit, only nine patients showed refractive changes >0.20 D; four had a hyperopic shift and 5 had a myopic shift. They, therefore, reported no significant association between blood sugar and RE, consistent with our findings. Since the cross-sectional survey observed a marginal difference in the baseline RE between the two diabetes groups (FBS ≤ 7 vs. FBS > 7) but not corroborated by the follow-up study, we recommend a larger population study for a thorough investigation of the possible association between blood sugar and RE.
Previous studies, including our earlier work, have found that diabetes was associated with a higher risk of ocular accommodation problems [5,6,7]. But it was not yet known whether regulating the blood glucose concentration help maintain or restore normal ocular accommodative functions in the general population of type-1 diabetes patients. This study is novel because it demonstrates a dynamic relationship between blood glucose concentration and ocular accommodative functions, which were previously unknown. We found that higher FBS (>7 mmol/l) was associated with lower AoA and greater LoA, which are signs of increased risk of near vision problems resulting in blurredness. The study went a step further to show the therapeutic effect of reducing hyperglycemia on ocular accommodation for a group of type-1 diabetes patients on average.
A potential cause of accommodative problems in diabetes is due to a neurological disorder of cranial nerve III (CN III palsy), often characterized by complete or marked loss of the accommodative amplitude as well as other ocular functions [24]. Clinically, finding asymmetry of accommodation often suggests CN III palsy. Another common cause for the loss of accommodation in the eye is related to structural lens changes. Studies have shown that biochemical changes, including variations in the enzyme activity of aldose reductase, sorbitol dehydrogenase, and the sorbitol levels, occurred in the lens of rodents with experimentally induced diabetes and impacted on the refractive property, curvature, and elasticity of the lens, causing changes in RE and accommodation [25, 26]. Similarly, it has been noted that in humans with type-1 diabetes these biochemical changes can be observed in the lens [27]. We, therefore, speculate that the reduction in AoA found in our diabetes patients might be related to the biochemical and structural changes in the crystalline lens, based on our observation that none had any asymmetry of accommodation or associated ocular neurological disorders such as unequal pupil sizes, eye deviations, and ptosis [24]. In addition, most of our study diabetes patients still preserved a significant level of accommodation despite the reduction.
Study limitations
Measurement of the AoA objectively by the MDR approach is a legitimate clinical method but is open to question in terms of accuracy in a research setting unless reliability is reported. This is because the change from a bright, full “with” reflex, to a narrow, slow dull one can be quite subjective and difficult to interpret unless the loss of accommodation is sudden and complete, so easier to see. To overcome the reliability problem, the examiner who is an experienced optometrist performed all procedures and the reproducibility recorded for the technique was acceptable.
We observed that the FBS readings recorded at baseline as well on the follow-up visit were very high in type-1 diabetes patients. In fact, 6 of the 15 patients having a reduction of their FBS during the follow-up visit still recorded FBS values >15 mmol/L while on insulin treatment. Despite this observation, we are unable to suggest that these type-1 diabetes patients were irresponsive to insulin treatment for the control of blood sugar. However, the challenge of noncompliance with insulin treatment is widely reported among diabetes patients not under in-patient supervision [28]. There is a limitation for the use of FBS for the monitoring of adequate control of blood sugar because it is affected by short-term lifestyle changes, including overactivity and stress [29]. That notwithstanding, a recent study found that there was a significant correlation between FBS and HbA1c [30], which measures the percentage of blood sugar attached to hemoglobin and has the advantage of giving the average blood sugar level for the past 2–3 months. Also, the outcomes of this study should be interpreted cautiously taking into consideration the age range of the participants, as it may not apply to older diabetes patients. Again, the findings of this study may not apply to the long-term changes that may occur as patients continued with treatment.
Conclusion
This study elucidates the underlying cause of near vision problems in type-1 diabetes by showing the dynamic state of the ocular accommodative system in response to changes in the blood sugar. Of clinical interest is the outcome that type-1 diabetic patients with improved blood sugar control had improved accommodative ability irrespective of the onset of the disease.
Summary
What was known before
-
Fluctuations in the distance and near vision are common in type-1 diabetes.
-
Treatment for type-1 diabetes includes insulin therapy to lower blood sugar.
What this study adds
-
The blood sugar level in type-1 diabetes affects the eye’s accommodative ability.
-
With adequate control of the blood sugar, it is possible to maintain or restore adequate ocular accommodation and to prevent near vision problems.
References
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the description of diabetes categories of glucose. Diabetes Care. 2003;26 Suppl 1:5–20.
American Diabetes Association. American Diabetes Association guidelines 2015. Clin Diabetes. 2015;33:97–111.
Wiemer NGM, Dubbelman M, Ringens PJ, Polak BCP. Measuring the refractive properties of the diabetic eye during blurred vision and hyperglycaemia using aberrometry and Scheimpflug imaging. Acta Ophthalmol. 2009;87:176–82.
García-Muñoz Á, Carbonell-Bonete S, Cacho-Martínez P. Symptomatology associated with accommodative and binocular vision anomalies. J Optom. 2014;7:178–92.
Braun CI, Benson WE, Remaley NA, Chew EY, Ferris FL. Accommodative amplitudes in the early treatment diabetic retinopathy study: ETDRS report number 21. Retina. 1995;15:275–81.
Khan A, Efron N, Mathur A, Edwards K, Pritchard N, Suheimat M. et al. Amplitude of accommodation in type 1 diabetes. Invest Ophthalmol Vis Sci. 2014;55:7014–8.
Abokyi S, Ilechie A, Asaam KA, Ntodie M. Fasting plasma sugar: a predictor of accommodative function in diabetes. Curr Eye Res. 2016;41:791–7.
Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, et al. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121:1141–7.
Yekta AA, Khabazkhoob M, Hashemi H, Ostadimoghaddam H, Ghasemi-Moghaddam S, Heravian J, et al. Binocular and accommodative characteristics in a normal population. Strabismus. 2017;25:5–11.
Slattery D, Amiel SA, Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med. 2018;35:306–16.
Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR. Day-to-day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care. 1999;22:394–8.
Rutstein RP, Fuhr PD, Swiatocha J. Comparing the amplitude of accommodation determined objectively and subjectively. Optom Vis Sci. 1993;70:496–500.
Rosenfield M, Chiu NN. Repeatability of subjective and objective refraction. Optom Vis Sci. 1995;72:577–9.
León AÁ, Medrano SM, Rosenfield M. A comparison of the reliability of dynamic retinoscopy and subjective measurements of amplitude of accommodation. Ophthalmic Physiol Opt. 2012;32:133–41.
Burns DH, Allen PM, Edgar DF, Evans BJW. Sources of error in clinical measurement of the amplitude of accommodation. J Optom. 2020;13:3–14.
Sayin N, Kara N, Nihat GP. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6:92–108.
Group TDC and CTR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in IDDM. N Engl J Med. 1993;329:977–86.
Reichard P, Nilsson BY, Rosenquist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–9.
Duke-Elder WS. Changes in refraction in diabetes mellitus. Br J Ophthalmol. 1925;9:167–87.
Lin SF, Lin PK, Chang FL, Tsai RK. Transient hyperopia after intensive treatment of hyperglycemia in newly diagnosed diabetes. Ophthalmologica. 2009;223:68–71.
Okamoto F, Sone H, Nonoyama T, Hommura S. Refractive changes in diabetic patients during intensive glycaemic control. Br J Ophthalmol. 2000;84:1097–102.
Sonmez B, Bozkurt B, Atmaca A, Irkec M, Orhan M, Aslan U. Effect of glycemic control on refractive changes in diabetic patients with hyperglycemia. Cornea. 2005;24:531–7.
Tai MC, Lin SY, Chen JT, Liang CM, Chou PI, Lu DW. Sweet hyperopia: refractive changes in acute hyperglycemia. Eur J Ophthalmol. 2006;16:663–6.
Watanabe K, Hagura R, Akanuma Y, Takasu T, Kajinuma H, Kuzuya N, et al. Characteristics of cranial nerve palsies in diabetic patients. Diabetes Res Clin Pract. 1990;10:19–27.
Kubo E, Maekawa K, Tanimoto T, Fujisawa S, Akagi Y. Biochemical and morphological changes during development of sugar cataract in Otsuka Long-Evans Tokushima Fatty (OLETF) rat. Exp Eye Res. 2001;73:375–81.
Bron AJ, Sparrow J, Brown NAP, Harding JJ, Blakytny R. The lens in diabetes. Eye. 1993;7:260–75.
Pierro L, Brancato R, Zaganelli E, Guarisco L, Calori G. Correlation of lens thickness with blood glucose control in diabetes mellitus. Acta Ophthalmol Scand. 1996;74:539–41.
Sarbacker GB, Urteaga EM. Adherence to insulin therapy. Diabetes Spectr. 2016;29:166–70.
Newsholme P, Cruzat V, Arfuso F, Keane K. Nutrient regulation of insulin secretion and action. J Endocrinol. 2014;221:R105–20.
Kam-On Chung J, Xue H, Wing-Hang Pang E, Chuen-Chu Tam D. Accuracy of fasting plasma glucose and hemoglobin A1c testing for the early detection of diabetes: a pilot study. Front Lab Med. 2017;1:76–81.
Acknowledgements
We express our profound gratitude to two diabetes physician specialists in the Cape Coast Teaching Hospital, Ghana, Dr Immanuel Amissah and Dr Nana Ama Barnes, for the support provided during data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abokyi, S., Ayerakwah, P.A., Abu, S.L. et al. Controlled blood sugar improves the eye’s accommodative ability in type-1 diabetes. Eye 35, 1198–1204 (2021). https://doi.org/10.1038/s41433-020-1058-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-1058-y