Abstract
Purpose
To investigate the risk factors associated with retinal detachment recurrence after first vitrectomy in high myopic eyes with macular hole retinal detachment (MHRD).
Methods
Patients with high myopic eyes with MHRD who underwent pars plana vitrectomy and silicone oil (SO) tamponade with a follow-up period more than 12 months and more than 3 months after SO removal were included in this retrospective study. Logistic regression was performed to determine the risk factors associated with retinal re-detachment.
Results
A total of 45 eyes from 43 patients were included in this study (11 male and 34 female patients). The retinal re-detachment rate after the first removal of silicon oil was 35.5% (16/45) in a mean postoperative follow-up time of 35.64 ± 32.94 months. Complete macular atrophy on fundus photography (odds ratio (OR) = 17.021, 95% confidence interval (95% CI): 2.218–130.609, p = 0.006) was a risk factor for MHRD after SO removal, while internal limiting membrane (ILM) peeling (OR = 0.091, 95% CI: 0.013–0.633, p = 0.015) and duration of SO tamponade (OR = 0.667, 95% CI: 0.454–0.980, p = 0.039) were protective factors.
Conclusion
For high myopic eyes with MHRD, complete macular atrophy was a significant risk factor for retinal re-detachment after silicon oil removal. ILM peeling and the duration of silicon oil tamponade were protective factors.
Similar content being viewed by others
Introduction
Macular hole retinal detachment (MHRD) occurs mainly in high myopic eyes. This condition is commonly seen in Asia, usually leading to a poor visual prognosis. Many surgical procedures to treat MHRD have been proposed, including macular sclera buckling [1], gas injection [2], vitrectomy with or without internal limiting membrane (ILM) peeling using gas or silicone oil (SO) as tamponade [3,4,5]. Recently, inverted ILM flaps or insertions and human amniotic membrane plugs as bridging tissues have been proposed as new methods to help close macular holes [6,7,8]. However, in highly myopic eyes with extremely long axial lengths (AL) or chorioretinal atrophy, surgical treatment can be difficult, and the long-term prognosis is still unclear. SO tamponade is usually recommended for eyes with marked macular chorioretinal atrophy to enhance retinal-choroid adhesion and improve the success rate [9].
In this study, we retrospectively reviewed highly myopic MHRD patients who had been treated with pars plana vitrectomy (PPV) and primary SO tamponade. All the patients had chorioretinal atrophy to various degrees. The study aimed to evaluate the surgical outcomes and investigate the prognostic factors associated with retinal detachment recurrence in these patients.
Methods
We reviewed the medical records of 43 patients with 45 eyes with retinal detachment owing to a myopic macular hole. All patients underwent PPV with SO tamponade between January 2005 and December 2018 at Peking University People’s Hospital. The inclusion criteria were as follows: (1) patients with a clinical presentation of retinal detachment caused by a macular hole; (2) refractive error ≥ −6.00 dioptres or AL ≥ 26 mm; (3) patients treated with PPV and primary SO tamponade; (4) a follow-up period of more than 12 months after primary vitrectomy and more than 3 months after SO removal; (5) medical information, including best-corrected visual acuity (BCVA), slit-lamp examination (SLE) and refraction test results, AL measurements by A-scan ultrasonography and colour fundus photography and optic coherence tomography (OCT) findings, was available before primary PPV; and (6) BCVA, SLE and OCT measurements were available. The exclusion criteria included (1) previous vitrectomy; (2) multiple retinal breaks; and (3) other existing retinal vascular diseases.
The information of each patient was collected, including age, sex, affected eye, BCVA, lens status, refraction, AL, presence of posterior staphyloma, presence of choroidal atrophy, presence of a dome-shaped macula, duration of SO tamponade, MH closure, retinal reattachment, and recurrent detachment.
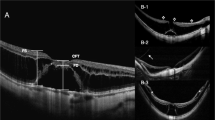
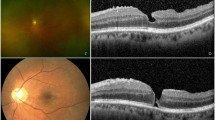
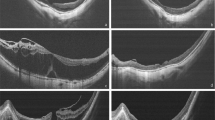
The degree of chorioretinal atrophy was defined according to the findings regarding fundus photograph 1 week after the first surgery based on a simplified classification system known as the International Photographic Classification and Grading System for myopic maculopathy (META-PM). Myopic chorioretinal atrophy is classified as a tessellated fundus (A1), diffuse chorioretinal atrophy (A2), patchy chorioretinal atrophy (A3) and complete macular atrophy (A4) based on fundus photography [10, 11]. Complete macular atrophy is recognised as a well-defined, round chorioretinal atrophic lesion on the fundus centred on the central fovea that has a round shape, whereas patchy chorioretinal atrophy is not centred on the fovea and has irregular margins. Complete choroidal atrophy on OCT was defined as a full-thickness defect of the choroidal vasculature within a 1 mm subfield of the central macula obtained 1 week after primary vitrectomy. Representative pictures are listed in Fig. 1.
a Diffuse chorioretinal atrophy (A2) appears yellowish-white at the posterior pole. b Patchy chorioretinal atrophy (A3) appears as well-defined, greyish-white lesions that are not centred on the fovea and have irregular margins. This image shows the unclosed macular hole after vitrectomy and the atrophic spot that is right next to the macular hole. c The eye has complete macular atrophy (A4) combined with staphyloma and severe posterior pole choroidal atrophy. d OCT shows the remaining choroidal vasculature under the central fovea. e Complete choroidal atrophy defined as a full-thickness defect of the choroidal vasculature within the central macula on OCT.
Standard 3-port PPV was performed under retrobulbar anaesthesia or general anaesthesia, followed by removal of the detached cortical vitreous. Indocyanine green staining and ILM peeling were performed in some patients depending on the surgeons. Fluid-air exchange and tamponade with SO (5000 centistokes) were used at the end of the surgical procedures. Cataract surgery was performed in patients with visually significant cataracts that precluded the view in order to perform vitrectomy. All patients were instructed to maintain a face-down position for 3 weeks. Patients were followed 1 day, 1 week and 1, 3, 6 and 12 months postoperatively. After SO removal, patients were followed after 1 day, 1 week and 1 month and then every 3 months thereafter. Macular hole closure was defined as the absence of a neurosensory defect over the fovea on OCT.
Statistics
Multiple factors related to MHRD recurrence, including age, average AL, AL > 30 mm, a dome-shaped macula, complete macular atrophy on fundus photography, complete choroidal atrophy on OCT 1 week after primary vitrectomy, ILM peeling, whether the macular hole was closed 1 month after primary vitrectomy and duration of SO tamponade, were analysed. If a clinical factor occurred infrequently (n < 5), the factor was excluded from the univariate analysis. All the patients were assigned to two groups according to whether MHRD recurred during the follow-up. Student’s t test or Mann–Whitney U test was used for continuous variables, and the Chi-square test or Fisher’s exact test was used for categorical variables. Selected clinical factors were subsequently entered in the multivariate analysis in an enter manner. Logistic regression analysis was performed using the enter method.
The BCVA was converted to the logarithm of the minimal angle of resolution (logMAR) for statistical analysis. Counting fingers, hand motion and light perception were classified as 1.85, 2.3 and 2.6 logMAR units, respectively [12]. p < 0.05 was considered significant.
Results
A total of 43 patients with 45 eyes were enrolled in this study (11 male and 34 female patients). The mean postoperative follow-up time was 35.64 ± 32.94 months. The mean age of all patients was 62.27 ± 8.87 years (median 65 years, range 40–78 years). The mean AL was 29.01 ± 2.07 mm. Forty-three eyes (95.5%) had posterior staphyloma. All the patients had chorioretinal atrophy to different degrees from A2 to A4. Seven (15.6%) eyes had a grade of A2, while 24 (53.3%) and 14 (31.1%) eyes had grades of A3 and A4, respectively. Eight eyes were pseudophakic before primary retinal surgery. Combined phacoemulsification was performed in four eyes, one eye underwent combined lensectomy, and 8 eyes underwent cataract surgery after removing the SO. Seven eyes received cataract surgery during the follow-up period after SO removal, seven eyes underwent cataract surgery during additional operations for retinal re-detachment, and the other ten eyes did not undergo cataract surgery. Thirty-one eyes experienced ILM peeling, while 14 eyes did not.
The mean BCVA (logMAR) before primary vitrectomy was 1.75 ± 0.40, and it increased to 1.39 ± 0.47 at the end of the follow-up. Seven eyes had a postoperative LogMAR BCVA better than 1.0. There was a significant difference in the BCVA before and after surgery (p < 0.001). The mean period of SO tamponade was 6.26 ± 5.50 months (median 5.0 months, range 1–30 months). Table 1 shows the demographics and clinical data of the enrolled subjects.
The anatomical success rate after the first vitrectomy was 100%. Seven eyes (15.6%) had retinal attachment with MH closure. The retinal re-detachment rate was 35.5% (16 eyes in 45 eyes) in total. After SO removal, two eyes (12.5%) had recurrent RD within 1 week, three eyes (18.3%) developed re-detachment between 1 week and 4 weeks later and five eyes (31.2%) had recurrence from 4 weeks to 12 months. Six identified cases of re-detachment (37.5%) were diagnosed after 1 year. Among these six eyes, two eyes had re-detachment at 3 years after SO removal, one eye at 4 years and one eye at 9 years. All of these 16 eyes had initial retinal attachment without MH closure. All recurrent patients underwent re-operations.
One eye underwent pneumatic retinopexy with 100% C3F8 tamponade, three underwent a second vitrectomy with C3F8 tamponade, while 12 eyes had SO tamponade. Four eyes still had SO tamponade at the end of the follow-up. One eye still had retinal detachment without SO tamponade at the last follow-up. The retinal reattachment rate at the last follow-up was 97.8% (44/45). Figure 2 summarises the clinical events of all patients in a visual manner. Figure 2a displays the timeline of recurrence and re-operation in all patients since the first surgery and the length of the entire follow-up. Figure 2b is arranged in the order of events, with no timeline displayed.
Figure a displays the timeline of recurrence and re-operation in all patients since the first surgery and the length of the entire follow-up. Figure b is arranged in the order of events, with no timeline displayed. All patients underwent silicone oil removal. Sixteen of 45 eyes developed retinal re-detachment, and the patients opted for second surgical interventions. A total of 37.5% of cases of re-detachment (six eyes) developed after 1 year. Four eyes still had SO tamponade at the end of the follow-up. One eye had retinal detachment without SO tamponade at the last follow-up. The final retinal reattachment rate was 97.8% (44/45).
The relationship among the risk factors associated with MHRD recurrence after SO removal in high myopic eyes is listed in Table 2. Complete macular atrophy on fundus photography, ILM peeling, duration of SO tamponade and macular hole closure showed significant differences between the two groups. Interaction analysis revealed no interaction between these factors, so they were all entered into logistic regression analysis as individual factors. Complete macular atrophy on fundus photography (odds ratio (OR) = 17.021, 95% confidence interval (95% CI): 2.218–130.609, p = 0.006) was a risk factor for MHRD after SO removal, while ILM peeling (OR = 0.091, 95% CI: 0.013–0.633, p = 0.015) and duration of SO tamponade (OR = 0.667, 95% CI: 0.454–0.980, p = 0.039) were protective factors. AL > 30 mm was not significantly associated with recurrence. Table 3 shows the results of logistic regression analysis of the clinical factors for recurrent retinal detachment.
Discussion
The preferred surgical treatment for MHRD remains controversial. Few randomised controlled studies have been designed to compare the outcomes of using gas versus SO tamponade due to difficulties with implementation. Nevertheless, it is known that PPV with primary SO tamponade shows a high retinal reattachment rate for treating MHRD, particularly in cases with macular atrophy. It has also been shown that PPV with primary SO tamponade can significantly improve postoperative BCVA [5, 13]. Recent studies on MHRD treated by PPV plus primary SO tamponade have suggested a high initial reattachment rate ranging from 76.2 to 96.4% [13,14,15,16]. Despite the promising reattachment rates reported, recurrence after SO removal is a significant factor for poor postoperative prognosis that should be taken into consideration in clinical settings. While several studies have revealed that SO removal time does not affect anatomical success [16,17,18], in our study, a longer duration of SO tamponade was indicated to be a protective factor against re-detachment. Since the duration varied over a wide range in this study, and complicated cases tended to have a longer duration of SO tamponade, the result should be interpreted with caution. Nevertheless, for patients with a large macular hole or extremely severe chorioretinal atrophy, a prolonged SO tamponade time should be considered to maintain retinal attachment. For those eyes with complete macular atrophy and very poor visual function, SO removal should not be considered if there are no serious SO-related complications because these patients have a very high risk of retinal re-detachment and are less likely to obtain visual improvement from SO removal.
It remains controversial whether ILM peeling is associated with the retinal reattachment rate. Some surgeons do not perform ILM peeling out of fear that the dye will enter the subretinal space and be potentially toxic to the photoreceptors; more importantly, abnormally thin retinas and chorioretinal atrophy also make this a risky procedure [16]. In Xie’s study, the retinal reattachment rate after initial SO removal with no ILM peeling was still 96%, although the MH closure rate was only 11.5% [16]. In Nakanishi’s study with gas tamponade, there was no significant difference in the anatomical success rate between ILM-peeled and ILM-preserved eyes [15]. In our study, a procedure without ILM peeling was confirmed to be a risk factor for retinal re-detachment. Fourteen eyes did not undergo ILM peeling because of the surgeons’ preferred practices, distortion or oedema of the cornea, lens opacity or severe chorioretinal atrophy. It was challenging to remove all the ILM from the extremely thin detached retina without surgical damage to the retina, particularly in very highly myopic eyes with significant chorioretinal atrophy [19].
Although chorioretinal atrophy is supposed to be a risk factor for re-detachment, few studies have discussed the relationship between the severity of chorioretinal atrophy and the recurrence rate. In Hong’s study [20], areas of patchy chorioretinal atrophy were not significantly associated with anatomical success. Nakanishi’s study showed that there was no difference in tigroid or deep atrophy between eyes with initial anatomical success and those with initial failure. However, all eyes had gas tamponade in this study [15]. Xie et al. classified posterior pole chorioretinal changes as atrophy or no atrophy, and these changes were not found to be associated with initial anatomical success after SO treatment [16].
In our study, we classified myopic chorioretinal atrophy according to the META-PM system. Due to the small number of subjects in each group after grouping, it is difficult to draw reliable conclusions by direct analysis. Therefore, we combined patients with A2 and A3 into one group (patients without complete macular atrophy) and compared them with patients with complete macular atrophy (A4). The results showed that complete macular atrophy was a risk factor for recurrence. We also tried to identify if there was a link between the degree of macular atrophy shown on OCT and recurrence. However, the results were not statistically significant, possibly because the small amount of residual choroidal vasculature structure shown on OCT was not sufficient to provide support for retinal adhesion. Therefore, we concluded that the presence, severity and location of macular atrophy should be evaluated and followed to help assess the risk of MHRD recurrence. Preoperative chorioretinal atrophy assessment can be difficult because of retinal detachment. According to a recent study, the gradual development of macular atrophy can occur in 11% of highly myopic eyes after PPV for MHRD [21]. Therefore, the evaluation should be conducted as soon as possible after retinal reattachment.
Teke et al. [17] reported RD development within the first 2 months after SO removal in 83.9% of cases (maximum 5.5 months). Laidlaw et al. found that 38 of 57 cases (66%) developed recurrent MHRD within the first 50 days after SO removal, while in two cases, MHRD was identified 5.5 months later [22]. Our study showed that 37.5% of cases of re-detachment (6 eyes) developed after 1 year, all of which had initial retinal attachment with MH open. One eye developed recurrent MHRD 9 years after the operation. Nevertheless, after the second surgery, the final retinal reattachment rate was up to 97.8%. Therefore, for highly myopic eyes with a persistently open MH, long-term follow-up is necessary to be cautious to the late recurrent MHRD.
Our study had the following limitations: a small sample size, a retrospective design and a lack of a randomised controlled study group. Further prospective controlled studies should be performed to confirm our conclusions.
In conclusion, complete macular atrophy was a significant risk factor for retinal re-detachment after SO removal. The presence of macular atrophy along with its degree and location of should be evaluated by fundus examination and fundus photography as soon as retinal reattachment is obtained. Long-term follow-up is highly recommended for all postoperative patients, particularly those with persistently open MHs, in the case of late RD recurrence.
Summary
What was known before
-
The preferred surgical treatment for MHRD remains controversial.
-
Recurrence after SO removal is a significant factor for poor postoperative prognosis for MHRD.
What this study adds
-
Complete macular atrophy is a significant risk factor for retinal re-detachment after SO removal.
-
The presence of macular atrophy should be evaluated by fundus examination and fundus photography as soon as the retina attained reattachment.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request. Informed consent for publication of the clinical dataset from participants has been obtained.
References
Sasoh M, Yoshida S, Ito Y, Matsui K, Osawa S, Uji Y. Macular buckling for retinal detachment due to macular hole in highly myopic eyes with posterior staphyloma. Retina. 2000;20:445–9.
Miyake Y. A simplified method of treating retinal detachment with macular hole. Am J Ophthalmol. 1984;97:243–5.
Wolfensberger TJ, Gonvers M. Long-term follow-up of retinal detachment due to macular hole in myopic eyes treated by temporary silicone oil tamponade and laser photocoagulation. Ophthalmology. 1999;106:1786–91.
Gonvers M, Machemer R. A new approach to treating retinal detachment with macular hole. Am J Ophthalmol. 1982;94:468–72.
Scholda C, Wirtitsch M, Biowski R, Stur M. Primary silicone oil tamponade without retinopexy in highly myopic eyes with central macular hole detachments. Retina. 2005;25:141–6.
Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–25.
Chen SN, Yang CM. Inverted internal limiting membrane insertion for macular hole-associated retinal detachment in high myopia. Am J Ophthalmol. 2016;162:99–106.e101.
Caporossi T, Pacini B, De Angelis L, Barca F, Peiretti E, Rizzo S. Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length of >30 mm. Retina. 2019. https://doi.org/10.1097/IAE.0000000000002699.
Chen YP, Chen TL, Yang KR, Lee WH, Kuo YH, Chao AN, et al. Treatment of retinal detachment resulting from posterior staphyloma-associated macular hole in highly myopic eyes. Retina. 2006;26:25–31.
Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–83.e7.
Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, Garcia-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80–115.
Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–40.
Nishimura A, Kimura M, Saito Y, Sugiyama K. Efficacy of primary silicone oil tamponade for the treatment of retinal detachment caused by macular hole in high myopia. Am J Ophthalmol. 2011;151:148–55.
Meng L, Wei W, Li Y, Han X, Shi X, Yang M. Treatment of retinal detachment secondary to macular hole in highly myopic eyes: pars plana vitrectomy with internal limiting membrane peel and silicone oil tamponade. Retina. 2014;34:470–6.
Nakanishi H, Kuriyama S, Saito I, Okada M, Kita M, Kurimoto Y, et al. Prognostic factor analysis in pars plana vitrectomy for retinal detachment attributable to macular hole in high myopia: a multicenter study. Am J Ophthalmol. 2008;146:198–204.
Xie A, Lei J. Pars plana vitrectomy and silicone oil tamponade as a primary treatment for retinal detachment caused by macular holes in highly myopic eyes: a risk-factor analysis. Curr Eye Res. 2013;38:108–13.
Teke MY, Balikoglu-Yilmaz M, Yuksekkaya P, Citirik M, Elgin U, Kose T, et al. Surgical outcomes and incidence of retinal redetachment in cases with complicated retinal detachment after silicone oil removal: univariate and multiple risk factors analysis. Retina. 2014;34:1926–38.
Choudhary MM, Choudhary MM, Saeed MU, Ali A. Removal of silicone oil: prognostic factors and incidence of retinal redetachment. Retina. 2012;32:2034–8.
Mancino R, Ciuffoletti E, Martucci A, Aiello F, Cedrone C, Cerulli L, et al. Anatomical and functional results of macular hole retinal detachment surgery in patients with high myopia and posterior staphyloma treated with perfluoropropane gas or silicone oil. Retina. 2013;33:586–92.
Hong N, Huang BS, Tong JP. Primary silicone oil tamponade and internal limiting membrane peeling for retinal detachment due to macular hole in highly myopic eyes with chorioretinal atrophy. BMC Ophthalmol. 2015;15:165.
Fang Y, Yokoi T, Shimada N, Du R, Shinohara K, Takahashi H, et al. Development of macular atrophy after pars plana vitrectomy for myopic traction maculopathy and macular hole retinal detachment in pathologic myopia. Retina. 2019. https://doi.org/10.1097/IAE.0000000000002709.
Laidlaw DA, Karia N, Bunce C, Aylward GW, Gregor ZJ. Is prophylactic 360-degree laser retinopexy protective? Risk factors for retinal redetachment after removal of silicone oil. Ophthalmology. 2002;109:153–8.
Acknowledgements
We thank Dong Chongya for his help in statistical analysis and American Journal Experts (AJE) for providing medical English editing for this manuscript.
Funding
The study was supported by Capital clinical diagnosis and treatment technology research and demonstration application project of China (Grant Z191100006619029) and the Research Fund for Science and Technology Program of Beijing (No. Z161100000516037). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was approved by the Peking University People’s Hospital Review Board and adhered to the tenets of the Declaration of Helsinki and Health Insurance Portability and Accountability Act.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, M., Tang, J., Jia, Z. et al. Long-term follow-up of primary silicone oil tamponade for retinal detachment secondary to macular hole in highly myopic eyes: a prognostic factor analysis. Eye 35, 625–631 (2021). https://doi.org/10.1038/s41433-020-0922-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0922-0