Abstract
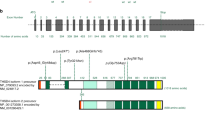
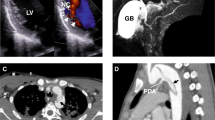
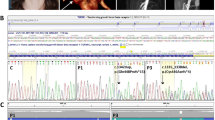
The ACTA2 gene codes for alpha-smooth muscle actin, a critical component of the contractile apparatus of the vascular smooth muscle cells. Autosomal dominant variants in the ACTA2 gene have been associated to familial non-syndromic thoracic aortic aneurysm/dissection (TAAD). They are thought to act through a dominant-negative mechanism. These variants display incomplete penetrance and variable expressivity, complicating the validation of ACTA2 variants pathogenicity by family segregation studies. In this study, we developed a yeast based assay to test putative TAAD-associated ACTA2 variants. We identified five new heterozygous ACTA2 missense variants in TAAD patients through next generation sequencing. We decided to test their pathogenicity in Saccharomyces cerevisiae, since yeast actin is very similar to human alpha-smooth muscle actin, and the residues at which the TAAD-associated variants occur in ACTA2 are well conserved. A wild type yeast strain was transformed with a vector expressing the different mutant alleles, to model the heterozygous condition of patients. Then, we evaluated yeast growth by spot test and cytoskeletal and mitochondrial morphology by fluorescence microscopy. We found that mutant yeast strains displayed only mild growth defects but a significant increase in the percentage of cells with abnormal mitochondrial distribution and abnormal organization of the actin cytoskeleton compared to controls. All variants appeared to interfere with the activity of wild type actin in yeast, suggesting a dominant-negative pathogenic mechanism. Our results demonstrate the utility of using the yeast actin model system to validate the pathogenicity of TAAD-associated ACTA2 variants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all supporting data are available either within the article or upon request to the corresponding author. The variants identified in this work have been submitted to ClinVar by our group (c.218T > C, SCV004024262; c.648G > C, SCV004024222; c.655T > C, SCV004024263), or previously submitted by others (c.78C > A, VCV000580143.10; c.79G > A, VCV001329101.4; c.352C > T, VCV001053987.14, and c.772C > T, VCV000018278.16).
References
Pollard TD. Actin and actin-binding proteins. Cold Spring Harb Perspect Biol. 2016;8:a018226. https://doi.org/10.1101/cshperspect.a018226.
Perrin BJ, Ervasti JM. The actin gene family: function follows isoform. Cytoskeleton. 2010;67:630–4. https://doi.org/10.1002/cm.20475.
Lehman W, Morgan KG. Structure and dynamics of the actin-based smooth muscle contractile and cytoskeletal apparatus. J Muscle Res Cell Motil. 2012;33:461–9. https://doi.org/10.1007/s10974-012-9283-z.
Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem. 1984;259:14383–8. https://doi.org/10.1016/S0021-9258(17)42610-X.
Lu H, Fagnant PM, Bookwalter CS, Joel P, Trybus KM. Vascular disease-causing pathogenic variant R258C in ACTA2 disrupts actin dynamics and interaction with myosin. Proc Natl Acad Sci USA. 2015;112:E4168–77. https://doi.org/10.1073/pnas.1507587112.
Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, et al. Pathogenic variants in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–93. https://doi.org/10.1038/ng.2007.6.
Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–27. https://doi.org/10.1016/j.ajhg.2009.04.007.
Regalado ES, Mellor-Crummey L, De Backer J, Braverman AC, Ades L, Benedict S, et al. Clinical history and management recommendations of the smooth muscle dysfunction syndrome due to ACTA2 arginine 179 alterations. Genet Med. 2018;20:1206–15. https://doi.org/10.1038/gim.2017.245.
Yuan SM. α-smooth muscle actin and ACTA2 gene expressions in vasculopathies. Braz J Cardiovasc Surg. 2015;30:644–9. https://doi.org/10.5935/1678-9741.20150081.
Arnaud P, Hanna N, Benarroch L, Aubart M, Bal L, Bouvagnet P, et al. Genetic diversity and pathogenic variants as possible predictors of severity in a French sample of nonsyndromic heritable thoracic aortic aneurysms and dissections (nshTAAD). Genet Med. 2019;21:2015–24. https://doi.org/10.1038/s41436-019-0444-y.
Milewicz DM, Carlson AA, Regalado ES. Genetic testing in aortic aneurysm disease: PRO. Cardiol Clin. 2010;28:191–7. https://doi.org/10.1016/j.ccl.2010.01.017.
Renard M, Callewaert B, Baetens M, Campens L, MacDermot K, Fryns JP, et al. Novel MYH11 and ACTA2 pathogenic variants reveal a role for enhanced TGFβ signaling in FTAAD. Int J Cardiol. 2013;165:314–21. https://doi.org/10.1016/j.ijcard.2011.08.079.
Mishra M, Huang J, Balasubramanian MK. The yeast actin cytoskeleton. FEMS Microbiol Rev. 2014;38:213–27. https://doi.org/10.1111/1574-6976.12064.
Ng R, Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1980;77:3912–6. https://doi.org/10.1073/pnas.77.7.3912.
Macchione F, Salviati L, Bordugo A, Vincenzi M, Camilot M, Teofoli F, et al. Multiple acyl-COA dehydrogenase deficiency in elderly carriers. J Neurol. 2020;267:1414–9. https://doi.org/10.1007/s00415-020-09729-z.
Giniger E, Varnum SM, Ptashne M. Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell. 1985;40:767–74. https://doi.org/10.1016/0092-8674(85)90336-8.
West RW, Yocum RR, Ptashne M. Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol Cell Biol. 1984;4:2467–78. https://doi.org/10.1128/mcb.4.11.2467-2478.1984.
Gietz RD, Woods RA. Yeast transformation by the LiAc/SS carrier DNA/PEG method. Methods Mol Biol 2006;313:107–20. https://doi.org/10.1385/1-59259-958-3:107.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. https://doi.org/10.1038/227680a0.
Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W, et al. Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:847–53. https://doi.org/10.1091/mbc.01-12-0588.
Westermann B, Neupert W. Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast. 2000;16:1421–7. https://doi.org/10.1002/1097-0061(200011)16:153.0.co;2-u.
Bergeron SE, Wedemeyer EW, Lee R, Wen KK, McKane M, Pierick AR, et al. Allele-specific effects of thoracic aortic aneurysm and dissection alpha-smooth muscle actin pathogenic variants on actin function. J Biol Chem. 2011;286:11356–69. https://doi.org/10.1074/jbc.M110.203174.
Writing Committee Members, Isselbacher EM, Preventza O, Hamilton Black J 3rd, Augoustides JG, Beck AW, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2023;166:e182–331. https://doi.org/10.1016/j.jtcvs.2023.04.023.
Devereux RB, de Simone G, Arnett DK, Best LG, Boerwinkle E, Howard BV, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110:1189–94. https://doi.org/10.1016/j.amjcard.2012.05.063.
Brownstein AJ, Ziganshin BA, Kuivaniemi H, Body SC, Bale AE, Elefteriades JA. Genes associated with thoracic aortic aneurysm and dissection: an update and clinical implications. Aorta. 2017;5:11–20. https://doi.org/10.12945/j.aorta.2017.17.003.
Johannes FJ, Gallwitz D. Site-directed mutagenesis of the yeast actin gene: a test for actin function in vivo. EMBO J. 1991;10:3951–8. https://doi.org/10.1002/j.1460-2075.1991.tb04965.x.
Liu Z, Chang AN, Grinnell F, Trybus KM, Milewicz DM, Stull JT, et al. Vascular disease-causing mutation, smooth muscle α-actin R258C, dominantly suppresses functions of α-actin in human patient fibroblasts. Proc Natl Acad Sci USA. 2017;114:E5569–78. https://doi.org/10.1073/pnas.1703506114.
Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, Pon LA. Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr Biol. 2004;14:1996–2004. https://doi.org/10.1016/j.cub.2004.11.004.
McKane M, Wen KK, Boldogh IR, Ramcharan S, Pon LA, Rubenstein PA. A mammalian actin substitution in yeast actin (H372R) causes a suppressible mitochondria/vacuole phenotype. J Biol Chem. 2005;280:36494–501. https://doi.org/10.1074/jbc.M506970200.
Drubin DG, Jones HD, Wertman KF. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993;4:1277–94. https://doi.org/10.1091/mbc.4.12.1277.
Parker F, Baboolal TG, Peckham M. Actin mutations and their role in disease. Int J Mol Sci. 2020;21:3371. https://doi.org/10.3390/ijms21093371.
An HS, Mogami K. Isolation of 88F actin mutants of Drosophila melanogaster and possible alterations in the mutant actin structures. J Mol Biol. 1996;260:492–505. https://doi.org/10.1006/jmbi.1996.0417.
Ilkovski B, Nowak KJ, Domazetovska A, Maxwell AL, Clement S, Davies KE, et al. Evidence for a dominant-negative effect in ACTA1 nemaline myopathy caused by abnormal folding, aggregation and altered polymerization of mutant actin isoforms. Hum Mol Genet. 2004;13:1727–43. https://doi.org/10.1093/hmg/ddh185.
Veitia RA. Exploring the molecular etiology of dominant-negative pathogenic variants. Plant Cell. 2007;19:3843–51. https://doi.org/10.1105/tpc.107.055053.
Regalado ES, Guo DC, Prakash S, Bensend TA, Flynn K, Estrera A, et al. Aortic disease presentation and outcome associated with ACTA2 mutations. Circ Cardiovasc Genet. 2015;8:457–64. https://doi.org/10.1161/CIRCGENETICS.114.000943.
Bartolák-Suki E, Imsirovic J, Nishibori Y, Krishnan R, Suki B. Regulation of mitochondrial structure and dynamics by the cytoskeleton and mechanical factors. Int J Mol Sci. 2017;18:1812. https://doi.org/10.3390/ijms18081812.
Shah M, Chacko LA, Joseph JP, Ananthanarayanan V. Mitochondrial dynamics, positioning and function mediated by cytoskeletal interactions. Cell Mol Life Sci. 2021;78:3969–86. https://doi.org/10.1007/s00018-021-03762-5.
Portelli SS, Hambly BD, Jeremy RW, Robertson EN. Oxidative stress in genetically triggered thoracic aortic aneurysm: role in pathogenesis and therapeutic opportunities. Redox Rep. 2021;26:45–52. https://doi.org/10.1080/13510002.2021.1899473.
Suslov AV, Afanasyev MA, Degtyarev PA, Chumachenko PV, Ekta MB, Sukhorukov VN, et al. Molecular pathogenesis and the possible role of mitochondrial heteroplasmy in thoracic aortic aneurysm. Life. 2021;11:1395. https://doi.org/10.3390/life11121395.
Calderan C. Development of yeast models for validating novel pathogenic mutations associated with human aortic aneurysm and primary Coenzyme Q10 deficiency. Research Padua Archive. 2023. https://hdl.handle.net/11577/3469813.
Acknowledgements
This research was part of a doctoral dissertation by CC with the title “Development of yeast models for validating novel pathogenic mutations associated with human aortic aneurysm and primary Coenzyme Q10 deficiency” [40].
Funding
This work was supported by a grant from “IRP—Città della Speranza” to LS.
Author information
Authors and Affiliations
Contributions
CC, MAD and LS conceived the study concept; LS acquired funding; US, ET and LS collected patients’ samples and clinical data, and performed the molecular diagnosis; CC performed functional studies and microscopic analysis; MAD, LP, GS and LS provided their expertise for the experiments; CC, MAD, GS and LS wrote and edited the manuscript; all authors reviewed and commented on the final draft manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was not needed in this study because we only used data from patients obtained in our diagnostic genetics unit. All genetic studies were performed with the informed consent of the patients which also accepted the use of results for research studies. All experiments were performed without employing biological material from the patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calderan, C., Sorrentino, U., Persano, L. et al. A yeast based assay establishes the pathogenicity of novel missense ACTA2 variants associated with aortic aneurysms. Eur J Hum Genet (2024). https://doi.org/10.1038/s41431-024-01591-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41431-024-01591-1