Abstract
Background
Serum albumin concentrations are frequently used to monitor nutritional therapy in the hospital setting but supporting studies are largely lacking. Within this secondary analysis of a randomized nutritional trial (EFFORT), we assessed whether nutritional support affects short-term changes in serum albumin concentrations and whether an increase in albumin concentration has prognostic implications regarding clinical outcome and response to treatment.
Methods
We analyzed patients with available serum albumin concentrations at baseline and day 7 included in EFFORT, a Swiss-wide multicenter randomized clinical trial that compared individualized nutritional therapy with usual hospital food (control group).
Results
Albumin concentrations increased in 320 of 763 (41.9%) included patients (mean age 73.3 years (SD ± 12.9), 53.6% males) with no difference between patients receiving nutritional support and controls. Compared with patients that showed a decrease in albumin concentrations over 7 days, those with an increase had a lower 180-day mortality [74/320 (23.1%) vs. 158/443 (35.7%); adjusted odds ratio 0.63, 95% CI 0.44 to 0.90; p = 0.012] and a shorter length of hospital stay [11.2 ± 7.3 vs. 8.8 ± 5.6 days, adjusted difference −2.2 days (95%CI −3.1 to −1.2)]. Patients with and without a decrease over 7 days had a similar response to nutritional support.
Conclusion
Results from this secondary analysis indicate that nutritional support did not increase short-term concentrations of albumin over 7 days, and changes in albumin did not correlate with response to nutritional interventions. However, an increase in albumin concentrations possibly mirroring resolution of inflammation was associated with better clinical outcomes. Repeated in-hospital albumin measurements in the short-term is, thus, not indicated for monitoring of patients receiving nutritional support but provides prognostic information.
Trail Registration
ClinicalTrials.gov Identifier: NCT02517476.
Similar content being viewed by others
Introduction
Historically, serum albumin concentration was considered to be a marker of nutritional status and physicians monitored albumin concentrations in patients during their hospital stay. This assumption was based on the pathophysiological grounds that albumin concentration reflects circulating proteins in plasma, with lower concentrations indicating nutritional deficiencies [1, 2]. However, it has been recognized for years that albumin as well as other visceral proteins (e.g., prealbumin) are markers of inflammation and correlate negatively with the severity of acute illness, but show little correlation with nutritional status [3,4,5,6,7]. In fact, albumin should be considered a negative acute-phase-protein, with concentrations declining in acute and chronic illness due to hepatic reprioritization of protein synthesis [2, 8, 9] and increased transcapillary escape of albumin [10]. In addition, albumin concentrations can fluctuate with hydration status [11, 12]. Yet, in clinical practice, many physicians still continue to monitor albumin concentrations to evaluate the response to nutritional support although supporting studies have been lacking [13].
Malnutrition is a common condition among medical inpatients, with a prevalence of about 30%, and is associated with increased mortality, morbidity, disability, and higher health care costs [14,15,16,17,18,19]. Several studies have shown that nutritional support reduces mortality as well as other adverse outcomes [19,20,21]. Therefore, it is important to early identify patients who are nutritionally at risk and provide them with appropriate nutritional therapy to reduce risks for clinical deterioration and, perhaps, improve outcomes. Nutritional screening based on a validated screening tool is the first step to identify patients at risk of malnutrition [17, 18, 22]. While screening tools are sensitive for diagnosis of malnutrition, they may not predict response to treatment [23]. More specific clinical parameters and blood biomarkers are needed to allow a more personalized approach to malnourished patients as not all patients show the same response to nutritional interventions. Recent studies have suggested that some nutritional biomarkers of inflammation, kidney function and muscle health, among others, predict treatment response to nutritional interventions and may help to personalize treatments. [14, 24,25,26,27] We recently found albumin concentrations measured in patients at hospital admission to be helpful to predict clinical outcomes among patients at nutritional risk, but albumin was not helpful in predicting treatment response to nutritional intervention [3]. Also, in the same trial, we measured prealbumin levels on admission, which has a shorter half-life as compared with albumin, but still only provided little information regarding nutritional treatment response [28]. In addition to baseline levels of these visceral proteins at hospital admission, there is still insufficient evidence regarding the usefulness of short-term changes in albumin concentrations over time to predict treatment response [2].
Herein, we tested the hypothesis that nutritional support influences short-term changes in serum albumin concentrations in medical inpatients and that these changes would correlate with medical outcomes and response to nutritional support in patients included in the Effect of early nutritional therapy on Frailty, Functional Outcomes, and Recovery on malnourished medical inpatients Trial (EFFORT) [19].
Material & Methods
Study design and setting
This is a secondary analysis of EFFORT [19], a pragmatic, multicenter, open-label, investigator-initiated trial performed in 8 Swiss hospitals from April 2014 to February 2018. The trial investigated the effect of early nutritional support versus standard hospital food on patient outcomes in medical inpatients. The study protocol was approved by the Ethics Committee of Northwestern Switzerland (EKNZ; 2014_001). All participants, or their authorized representatives, provided written informed consent. EFFORT was registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02517476). Detailed information about rationale, design as well as the results of the trial have been published elsewhere [19, 29].
Patient population and management
EFFORT enrolled adult ( ≥ 18 years of age) medical inpatients at nutritional risk with an anticipated hospital stay of at least 5 days who were willing to give informed consent within the first 48 hours after admission. Nutritional risk was defined as a Nutritional Risk Screening (NRS 2002) score of 3 points or more. The NRS 2002 consists of two parts: the patient’s current nutritional status and the severity of the underlying disease. Both parts score from 0 (absent) to 3 (severe) with an extra point for age ≥ 70 years. An total score of 3 or more points indicates “nutritionally at risk” and additional nutritional support should be considered [18, 30, 31]. More detailed information about NRS 2002 is provided in the Supplement. Patients were excluded if they were initially admitted to intensive care or surgical units, were incapable of ingesting food orally, had contraindications to nutritional supplements, were already receiving nutritional support at admission, were previously included in the study, had a terminal condition, anorexia nervosa, acute pancreatitis, acute liver failure, cystic fibrosis, stem cell transplantation or bariatric surgery. Participants were randomly assigned (1:1) by an interactive web-system to receive either individual nutritional therapy (intervention group) or standard hospital food (control group). As for the intervention group, individualized nutritional support was established within the first 48 h after admission. Energy and protein goals were calculated by a trained nutritionist who then developed an individual treatment plan for each patient. The initial approach was to use nutritional support by the oral route. If patients did not reach 75% of their protein and energy goals within 5 days, therapy could be escalated to enteral tube or parenteral feeding. Upon admission, several other parameters including Barthel’s index [32] were collected according the trial protocol. Trained study nurses conducted a structured telephone interview to systematically assess predefined health-related outcomes 30 and 180 days after discharge.
Research objective and outcomes
We had three main goals for this analysis: first, to investigate how nutritional therapy impacts on the short-term changes in serum albumin concentrations from baseline to 7 days in the overall population and within subgroups of patients with high and low baseline albumin concentrations ( < 30 g/L or ≥ 30 g/L) [3, 33]. We used the same cut-offs for albumin as used in our previous publication [3] and which corresponds to normal values based on the assay used for measurement. Second, we aimed to investigate whether changes in serum albumin concentrations would predict clinical and functional outcomes and, thirdly, response to nutritional support. To investigate these hypotheses, we calculated several models in the overall population and further stratified patients based on their inflammatory status, i.e., based on their levels of CRP similar to a previous analysis [27]. Stratification was done low CRP ( < 100 mg/l) and high CRP ( ≥ 100 mg/l) on admission.
Our primary endpoint for the prognostic analyses was long-term all-cause mortality measured over 180 days, while for the response to nutritional support we focused on short-term 30-day mortality. Secondary endpoints where adverse outcome within 30 days (composite endpoint consisting of all-cause mortality, admission to the intensive care unit from medical ward, major complications (nosocomial infection, respiratory failure, major cardiovascular event, acute renal failure, gastrointestinal failure), nonelective hospital readmission after discharge, decline in functional status ≥10% measured by Barthel’s index), length of hospital stay, loss of function according to Barthel’s index (score ranging from 0 to 100 with lower scores indicating worse functional status) and quality of life measured by 5-level European Quality of life 5 Dimensions index (EQ5D) including the self-assessment visual analogue scale (VAS). Detailed information for the single endpoints and their composites is summarized in the Supplement. We defined treatment response as the difference in outcomes among control group and intervention group patients, similar to the initial EFFORT trial.
Statistical analyses
Continuous variables are shown as means and standard deviation. Categorical and binary data are expressed as counts and percentages. Baseline characteristics were compared between patients with an increase in albumin and without an increase in albumin after 7 days using Pearson’s χ2 test for binary and categorical variables and Student t-test for continuous variables. We also studied the association of an increase in serum albumin with different clinical outcomes in regression analysis. We used logistic regression with odds ratios (OR) and 95% confidence interval (CI) for binary outcomes and linear regression with coefficient (Coef) and 95% confidence interval (CI) for continuous variables. All analyses were adjusted for the following predefined covariates: age, sex, main diagnosis, comorbidities and study center. Statistical analyses were performed with STATA 15.1 (Stata Corp, College Station, TX, USA).
Results
Patient population
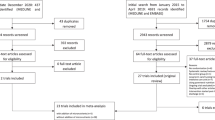
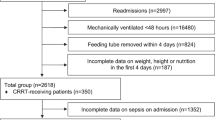
From the 2088 participants of the initial trial, we had complete data on 763 patients regarding baseline and day 7 albumin concentration and all clinical outcomes (Fig. 1). Mean age was 73 ( ± 13) years and 54% of participants were male. A total of 320 participants showed an increase in serum albumin concentration after 7 days (delta albumin from baseline to day 7), while 443 showed a decrease. Patients with albumin increase had a higher body mass index on admission and had differences regarding the main admission diagnosis and types of comorbidities compared with patients with no albumin increase. Table 1 shows baseline characteristics for the overall trial cohort and stratified by increase or decrease in albumin after 7 days.
An additional stratification by low C-reactive protein (CRP) ( < 100 mg/l) and high CRP ( ≥ 100 mg/l) on admission is provided in the Supplement (Table 1).
Change in albumin concentration from baseline to day 7 in patients with and without nutritional intervention
Table 2 shows albumin concentrations at baseline and day 7 in patients with and without nutritional support. Mean serum albumin concentration in the control group at baseline was 27.9 g/L and dropped slightly by 0.72 g/L to 27.2 g/L. Results in the nutritional intervention group were similar with a drop from 27.7 g/L to 26.9 g/L. There was no difference between intervention and control group patients regarding changes in albumin concentration [mean difference −0.06 g/L (95%CI −0.55 to 0.44)]. A corresponding analysis stratified for normal or low baseline albumin concentrations was similar but there was a slight higher proportion of patients with an albumin increase in the control group (81% vs. 63.3%, p = 0.002), a finding that remained significant in the adjusted regression analysis. We also performed additional stratification by low and high baseline CRP concentrations, which again showed similar results, but in patients with higher inflammation there was a stronger increase in albumin over time without differences among treatment groups (Supplement Tables 2.1, 2.2).
Association of kinetics of serum albumin level and clinical outcomes
We then investigated the prognostic value of changes in albumin concentrations regarding different clinical and functional outcomes (Table 3). Overall, the short-term changes in albumin concentrations were highly predictive for different short- and long-term clinical outcomes of patients. Participants with an increase in albumin showed a significantly reduced 180-days mortality [74/320 (23.1%) vs. 158/443 (35.7%); adjusted OR 0.63, 95% CI 0.44 to 0.9; p = 0.012)] and a reduced length of hospital stay (8.8 days vs. 11.16 days; adjusted difference −2.16 days, 95% CI −3.14 to 1.18; p < 0.001)]. Figure 2 shows the Kaplan-Meier-estimate for all-cause mortality within 180 days. When additionally stratifying by CRP concentrations, most results remained robust except for mortality in the low CRP group (Supplement Table 3.1–3.3).
Predictors for changes in albumin concentrations from baseline to day 7
Further, we investigated which factors were associated with an increase in albumin concentrations from baseline to day 7 in a linear regression analysis (Table 4). In the univariate model, we found several factors that were associated with an increase in albumin including higher protein and energy intake, and different admission diagnoses. When adjusting for baseline albumin and CRP concentrations, those results remained robust.
Effect of nutritional intervention in association with kinetics of serum albumin levels
Last, we evaluated whether the effectiveness of nutritional support concerning 30-day mortality in the intervention group and control group would differ according to changes in albumin concentrations. The mortality benefit of nutritional support was independent of changes in albumin concentrations in the overall population (p interaction 0.327), and also when stratified by albumin concentrations (Fig. 3).
Discussion
In this secondary post-hoc analysis of a randomized clinical trial, we investigated first whether nutritional support affects short-term changes in serum albumin concentrations among medical inpatients at nutritional risk, and second, whether an increase in albumin concentrations has prognostic implications regarding clinical outcome and treatment response. We found that 42% of patients in our study population had an increase in albumin concentrations from baseline to day 7 and, compared with usual hospital food, nutritional support was not associated with a more pronounced increase in albumin concentrations. The changes in serum albumin concentrations, however, provided prognostic information, and mortality and length of hospital stay were significantly lower in patients with an increase in albumin concentration compared with those with decrease. Finally, patients with and without increase in albumin concentrations had a similar treatment response to the nutritional intervention and monitoring of albumin from baseline to day 7 was, thus, not helpful for predicting response to nutritional support.
These findings are largely in line with a recent statement by the American Society for Parenteral and Enteral Nutrition (ASPEN) stating that “visceral proteins have not been shown to be sensitive markers of energy and protein intake adequacy and therefore should not be a guide for therapeutic changes.” [2] We observed that protein and energy intake was associated with increase in albumin concentration in the univariate model but not the multivariate model in this study. This may be due to direct effects of nutrition on albumin concentration and secondary effects as an improvement in appetite with leading to more food intake is also typically seen when the underlying disease improves. Thus, higher intake as a surrogate for better health, may have confounded the analysis.
While nutritional intervention did not show differences in albumin concentrations in the overall population suggesting that nutritional support had little effect on the short-term changes in serum albumin concentrations over one week, there were some significant effects in the subgroup of patients in participants with a high vs. normal albumin concentration at baseline ( ≥ 30 g/L) and low levels of the inflammatory marker CRP. We have previously reported that inflammation as assessed by CRP is an important predictor for the effect of nutrition on health outcomes and patients with low inflammation had most benefits [27]. About 25% of these patients had inflammation due to an infection, and the increase in albumin concentration over time in these patients may be due to the resolution of inflammation with subsequent increases in albumin concentrations, and rather than nutritional effects.
Interestingly, most patients in our study showed an absolute decrease in serum albumin concentrations in the short-term follow-up over 7 days. We assume that this was due to the acute disease of our patients with an increase in catabolism and the fact that human serum albumin has a half-life of about 19 days [34]. Therefore, the 7-day-course of our analysis was too short and longer-term follow-up may show an increase in concentrations at a later time point when the acute disease and inflammation has resolved. It would have been interesting to also look into prealbumin kinetics over 7 days for comparison, which, however was not possible due to missing day 7 data for prealbumin [28].
We are aware of several strengths and limitations. To our knowledge, this analysis is among the first and maybe the most comprehensive study to look at changes in serum albumin concentrations in a large population of medical patients from a previous randomized trial with detailed information about nutritional intake and the resolution of the disease. In the 1980s Winkler et al. [35]. as well as Ota et al. [36]. found prealbumin to be a better indicator for response to short-term nutritional support than other visceral proteins including albumin, due to the shorter half-life, in patients undergoing surgery and those with cancer, respectively. Since then, most studies focused on prealbumin and other visceral proteins as monitoring-parameters for nutritional therapy and little attention has been paid to albumin [37]. Unfortunately, we did not measure prealbumin concentrations over time, but only had admission levels measured in a subset of patients [28]. Thus, it is not possible within this analysis to compare albumin to prealbumin regarding prognostic implications. Other important limitations include possible selection bias due to the underlying trial and because only patients with two albumin concentrations (baseline and day 7) were included. There is also risk for residual confounding although we did adjust our analysis for important confounders. Thus, we excluded some patients who died within the first week or were discharged home early. Also, with 763 patients, our study is larger compared to previous trials but may still be underpowered to find small differences in clinical response in patients with low and normal prealbumin levels. Overall, as a secondary analysis, our findings are hypothesis-generating and need validation in prospective studies.
Conclusion
Results from this secondary analysis including medical inpatients at nutritional risk indicate that nutritional support did not increase concentrations of albumin within 7 days, and changes in serum albumin concentrations did not correlate with treatment response to nutritional interventions. However, an increase in albumin concentrations possibly mirroring resolution of inflammation was associated with better clinical outcomes. Repeated in-hospital albumin measurements in the short-term is, thus, not indicated for monitoring of patients receiving nutritional support but provides prognostic information.
Data availability
We intend to make data collected for the study, including anonymized individual participant data and a data dictionary defining each field in the set, available to others. Related documents will be available, including the trial protocol and the statistical analysis plan. These data will be available with the publication of our main manuscript and all secondary projects as outlined in our trial protocol on receipt of a letter of intention detailing the study hypothesis and statistical analysis plan. The steering committee of this trial will discuss all requests and decide on the basis of the scientific rigor of the proposal whether data sharing is appropriate. All applicants are asked to sign a data access agreement. Please send any request to the principal investigator of this trial.
References
Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enter Nutr. 1977;1:11–22. https://doi.org/10.1177/014860717700100101. e-pub ahead of print 1977/01/01
Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pr. 2021;36:22–28. https://doi.org/10.1002/ncp.10588. e-pub ahead of print 20201030
Bretschera C, Boesiger F, Kaegi-Braun N, Hersberger L, Lobo DN, Evans DC, et al. Admission serum albumin concentrations and response to nutritional therapy in hospitalised patients at malnutrition risk: Secondary analysis of a randomised clinical trial. EClinicalMedicine. 2022;45:101301 https://doi.org/10.1016/j.eclinm.2022.101301. e-pub ahead of print 20220211
Eckart A, Struja T, Kutz A, Baumgartner A, Baumgartner T, Zurfluh S, et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am J Med. 2020;133:713–22 e717. https://doi.org/10.1016/j.amjmed.2019.10.031. e-pub ahead of print 20191118
Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–64. https://doi.org/10.1016/j.jada.2004.05.213. e-pub ahead of print 2004/07/29
Shenkin A. Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition. Clin Chem. 2006;52:2177–9. https://doi.org/10.1373/clinchem.2006.077412
Kudsk KA, Tolley EA, DeWitt RC, Janu PG, Blackwell AP, Yeary S, et al. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. JPEN J Parenter Enter Nutr. 2003;27:1–9. https://doi.org/10.1177/014860710302700101
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. https://doi.org/10.1056/NEJM199902113400607. e-pub ahead of print 1999/02/11
Peterson VM, Moore EE, Jones TN, Rundus C, Emmett M, Moore FA, et al. Total enteral nutrition versus total parenteral nutrition after major torso injury: attenuation of hepatic protein reprioritization. Surgery. 1988;104:199–207. e-pub ahead of print 1988/08/01
Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781–4. https://doi.org/10.1016/s0140-6736(85)91447-3. e-pub ahead of print 1985/04/06
Lobo DN, Bjarnason K, Field J, Rowlands BJ, Allison SP. Changes in weight, fluid balance and serum albumin in patients referred for nutritional support. Clin Nutr. 1999;18:197–201. https://doi.org/10.1016/s0261-5614(99)80070-3
Lobo DN, Stanga Z, Simpson JA, Anderson JA, Rowlands BJ, Allison SP. Dilution and redistribution effects of rapid 2-litre infusions of 0.9% (w/v) saline and 5% (w/v) dextrose on haematological parameters and serum biochemistry in normal subjects: a double-blind crossover study. Clin Sci (Lond). 2001;101:173–9.
Lee ZY, Heyland DK. Determination of Nutrition Risk and Status in Critically Ill Patients: What Are Our Considerations? Nutr Clin Pr. 2019;34:96–111. https://doi.org/10.1002/ncp.10214. e-pub ahead of print 20181123
Schuetz P, Seres D, Lobo DN, Gomes F, Kaegi-Braun N, Stanga Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet. 2021;398:: 1927–38. https://doi.org/10.1016/S0140-6736(21)01451-3. e-pub ahead of print 20211014
Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, Miller S, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enter Nutr. 2014;38:186–95. https://doi.org/10.1177/0148607113512154. e-pub ahead of print 20131118
Felder S, Lechtenboehmer C, Bally M, Fehr R, Deiss M, Faessler L, et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. 2015;31:1385–93. https://doi.org/10.1016/j.nut.2015.06.007. e-pub ahead of print 20150720
Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21. https://doi.org/10.1016/s0261-5614(03)00098-0. Educational et al
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc EWG. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–36. https://doi.org/10.1016/s0261-5614(02)00214-5
Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393:2312–21. https://doi.org/10.1016/S0140-6736(18)32776-4. e-pub ahead of print 20190425
Gomes F, Baumgartner A, Bounoure L, Bally M, Deutz NE, Greenwald JL, et al. Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2:e1915138. https://doi.org/10.1001/jamanetworkopen.2019.15138. e-pub ahead of print 20191101
Kaegi-Braun N, Faessli M, Kilchoer F, Dragusha S, Tribolet P, Gomes F, et al. Nutritional trials using high protein strategies and long duration of support show strongest clinical effects on mortality. Results of an updated systematic review and meta-analysis. Clin Nutr Espen. 2021;45:45–54. https://doi.org/10.1016/j.clnesp.2021.08.003. e-pub ahead of print 20210824
Reber E, Gomes F, Vasiloglou MF, Schuetz P, Stanga Z. Nutritional risk screening and assessment. J Clin Med. 2019;8:1065. https://doi.org/10.3390/jcm8071065. e-pub ahead of print 20190720
Stalder L, Kaegi-Braun N, Gressies C, Gregoriano C, Tribolet P, Lobo DN, et al. Prospective validation of five malnutrition screening and assessment instruments among medical inpatients: Secondary analysis of a randomized clinical trial. Clin Nutr. 2022;41:1307–15. https://doi.org/10.1016/j.clnu.2022.04.025. e-pub ahead of print 20220426
Bargetzi A, Emmenegger N, Wildisen S, Nickler M, Bargetzi L, Hersberger L, et al. Admission kidney function is a strong predictor for the response to nutritional support in patients at nutritional risk. Clin Nutr. 2021;40:2762–71. https://doi.org/10.1016/j.clnu.2021.03.013. e-pub ahead of print 20210315
Kaegi-Braun N, Tribolet P, Baumgartner A, Fehr R, Baechli V, Geiser M, et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am J Clin Nutr. 2021;114:731–40. https://doi.org/10.1093/ajcn/nqab042
Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8:775 https://doi.org/10.3390/jcm8060775. e-pub ahead of print 20190531
Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kagi-Braun N, et al. Association of Baseline Inflammation With Effectiveness of Nutritional Support Among Patients With Disease-Related Malnutrition: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2020;3:e200663. https://doi.org/10.1001/jamanetworkopen.2020.0663. e-pub ahead of print 20200302
Bretscher C, Buergin M, Gurzeler G, Kagi-Braun N, Gressies C, Tribolet P, et al. Association between prealbumin, all-cause mortality, and response to nutrition treatment in patients at nutrition risk: Secondary analysis of a randomized controlled trial. JPEN. J Parenter Enter Nutr. 2023;47:408–19. https://doi.org/10.1002/jpen.2470. e-pub ahead of print 20230127
Schuetz P, Fehr R, Baechli V, Geiser M, Gomes F, Kutz A, et al. Design and rationale of the effect of early nutritional therapy on frailty, functional outcomes and recovery of malnourished medical inpatients trial (EFFORT): a pragmatic, multicenter, randomized-controlled trial. Int J Clin Trials. 2018;5:142–50. https://doi.org/10.18203/2349-3259.ijct20182085
Efthymiou A, Hersberger L, Reber E, Schonenberger KA, Kagi-Braun N, Tribolet P, et al. Nutritional risk is a predictor for long-term mortality: 5-Year follow-up of the EFFORT trial. Clin Nutr. 2021;40:1546–54. https://doi.org/10.1016/j.clnu.2021.02.032. e-pub ahead of print 20210304
Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, Baechli V, et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr. 2020;39:2720–9. https://doi.org/10.1016/j.clnu.2019.11.041. e-pub ahead of print 20191214
Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. e-pub ahead of print 1965/02/01
Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021;40:4745–61. https://doi.org/10.1016/j.clnu.2021.03.031. e-pub ahead of print 20210419
Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. 2015;12:793–812. https://doi.org/10.1517/17425247.2015.993313. e-pub ahead of print 20141218
Winkler MF, Gerrior SA, Pomp A, Albina JE. Use of retinol-binding protein and prealbumin as indicators of the response to nutrition therapy. J Am Diet Assoc. 1989;89:684–7. e-pub ahead of print 1989/05/01
Ota DM, Frasier P, Guevara J, Foulkes M. Plasma proteins as indices of response to nutritional therapy in cancer patients. J Surg Oncol. 1985;29:160–5. https://doi.org/10.1002/jso.2930290306. e-pub ahead of print 1985/07/01
Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enter Nutr. 2012;36:197–204. https://doi.org/10.1177/0148607111413896. e-pub ahead of print 20110728
Acknowledgements
We thank all patients and hospital staff for their support of our trial.
Funding
The initial trial was supported by grants from the Swiss National Science Foundation to PS and the Research Council of Kantonsspital Aarau, Switzerland. Unrelated to this trial, Nestlé Health Science and Abbott Nutrition previously provided unrestricted grant money to the institution of PS; The institution of ZS received speaking honorariums and research support from Nestlé Health Science, Abbott Nutrition, and Fresenius Kabi. All other authors report no conflicts of interest. Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Contributions
FB and PS were responsible for the data analysis and interpretation of the present secondary analysis. FB, DNL, and PS drafted the final manuscript and all authors contributed to the critical revision of the manuscript. PS was responsible for obtaining funding. PS, ZS, and BM were involved in obtaining funding, drafting the trial protocol, supervising study sites, drafting the final manuscript of the original EFFORT trial and approving the final version of the current manuscript. The corresponding authors had full access to all the data used and shared the final responsibility for the accuracy of the analyzed data. All authors were involved in data collection and/or interpretation and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boesiger, F., Poggioli, A., Netzhammer, C. et al. Changes in serum albumin concentrations over 7 days in medical inpatients with and without nutritional support. A secondary post-hoc analysis of a randomized clinical trial. Eur J Clin Nutr 77, 989–997 (2023). https://doi.org/10.1038/s41430-023-01303-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01303-w