Abstract
Background
Many people in modern society have insufficient exposure to ultraviolet B (UVB) sunlight, which may lead to vitamin D deficiency. We aimed to investigate the effect of a proto-type wearable light-emitting diode (LED) device emitting UVB light on serum 25-hydroxyvitamin D levels.
Methods
A total of 136 healthy adults were randomly assigned to receive either an active device emitting UVB light with a peak wavelength of 285 nm (n = 64) or a sham device emitting visible light (n = 72). All participants wore the device for a total of two minutes, one minute on each forearm, every day for 4 weeks. Serum 25-hydroxyvitamin D levels were assessed at baseline, 2, and 4 weeks of intervention, and 2 weeks after the end of the intervention.
Results
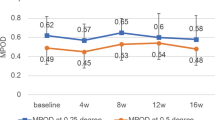
A significant difference was found between the experimental and control groups in changes in serum 25-hydroxyvitamin D levels from baseline after two (0.25 ± 3.10 ng/mL vs. −1.07 ± 2.68 ng/mL, p = 0.009) and 4 weeks of intervention (0.75 ± 3.98 ng/mL vs. −1.75 ± 3.04 ng/mL, p < 0.001). In the experimental group, the dropout rate due to mild, self-limiting adverse skin reactions was 11.8% (9/76). The mean total 25-hydroxyvitamin D production after UVB exposure was estimated at 0.031 ng/mL per 1 cm2 of skin area.
Conclusions
A prototype wearable LED UVB device was effective for improving 25-hydroxyvitamin D status. The development of a safer wearable LED device for phototherapy may provide a novel daily, at-home option for vitamin D supplementation.
Similar content being viewed by others
Introduction
Vitamin D is a nutrient responsible for many physiological functions, and inadequate vitamin D nutritional status is associated with various chronic diseases [1]. In humans, vitamin D can be synthesized in the skin via exposure to ultraviolet B (UVB: 280–320 nm wavelength) in sunlight [2], and cutaneous production is the primary natural source of vitamin D [3]. However, in modern society, many people have insufficient exposure to UVB radiation from sunlight due to the widespread use of sunscreen [4], aging [5], season and latitude [6], and indoor-oriented lifestyles [7], leading to worldwide inadequate vitamin D nutritional status [8].
A number of previous studies reported a meaningful improvement of vitamin D nutritional status after UVB light therapy for skin disorders without serious adverse reactions [9,10,11]. However, these currently used in-hospital phototherapies may not be suitable for the treatment of vitamin D deficiency due to high cost and inconvenience. Therefore, there is a need to develop a more convenient and efficient UVB light-emitting device for home phototherapy to improve vitamin D nutritional status.
A light-emitting diode (LED) is a semiconductor light-emitting source widely used in various industries, including in the field of medical devices [12]. LEDs have many advantages over other electronic light-emitting components such as energy efficiency, robustness, long lifetime, and, most of all, smaller size, which enables the fabrication of compact illuminating systems [13]. These features of LEDs also make them suitable for the production of relatively small and light, wearable light-emitting devices for daily use at home.
An animal study showed that the exposure conditions with ultraviolet UVB LEDs can achieve optimal vitamin D bio-fortification in pig skin [14]. However, data regarding the effect of UVB-emitting LED devices in producing vitamin D in the human skin are limited. A recent study reported that LED light was 2.4 times more efficient than sunlight in cutaneous vitamin D production [15]. Another study also showed that UVB-LED was effective in generating vitamin D in human skin [16]. However, these studies were based on in vitro experiments with ampules or human skin samples. To our knowledge, there has been no randomized controlled study to prove the effect of LED phototherapy devices on vitamin D nutritional status.
In this work, we aimed to examine the effect of a proto-type wearable LED UVB light-emitting device on vitamin D nutritional status of healthy adults. We conducted a randomized, single-blind, sham-controlled clinical trial with a relatively large study sample size.
Materials and methods
Study population
This study was conducted at the sleep clinic of Seoul National University Bundang Hospital (SNUBH), Seongnam, Korea. Healthy adults, aged 20–65 years, were recruited by advertisements in the local community from August 2020 to August 2021. The enrolled participants underwent screening tests at the first visit, which was within a week prior to the initiation of intervention. Those who experienced adverse skin reactions in the screening test were excluded. We also excluded those who met the following criteria: (1) history of light therapy or vitamin D supplementation within two months prior to study entry; (2) skin diseases including skin cancers and photosensitivity; (3) medical illnesses such as malignancy, respiratory diseases, infectious diseases, hepatic or renal impairment, and head trauma; and (4) psychiatric disorders including mood disorders, anxiety disorders, sleep disorders, and psychotic disorders. Written informed consent was obtained from all participants before the initiation of the study. This study was approved by the Institutional Review Board of SNUBH (B-2002-597-003) and was registered with the Clinical Research Information Service (CRIS), Republic of Korea (registration number KCT0007033).
Intervention
We used two different types of proto-type wearable light-emitting devices (the active and sham device), which were custom-designed at the School of Electrical Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea. The active device was composed of 48 LEDs (QD Jason, China, Model J35ABA285P09A) that emitted UVB light at a peak wavelength of 285 nm with the full width at a half-maxima of 11 nm. LEDs were arranged on a flexible printed circuit board (FPCB) in a 6 × 8 matrix format with an interval of 13 mm between adjacent LEDs. Then, the FPCB with these 48 UVB LEDs was placed in a 3-D printed housing that could be wrapped around the lower or upper arm. The distance between an LED and the surface of the skin under illumination was maintained at ~5 mm to ensure that light from the LEDs could spread over the skin. Furthermore, a clear elastomer of polydimethylsiloxane (PDMS) was placed between the LEDs and the surface of the skin to ensure that the skin-to-LED distance remained constant. One side of the PDMS layer was micro-structured to further enhance the spread of the LED light. The overall illuminated area was ~80 cm2. The light-diffusing structure could sustain a dose of ~7.8 mJ/cm2 per one-min exposure. The sham devices had an identical construction except that the LEDs emitted blue light with a peak wavelength of 465 nm instead of UVB light (Fig. 1).
At the first visit, a screening test was conducted using a testing device made of a single LED, which emitted UVB light with the same wavelength and intensity as the active device, onto a 3-cm2 area of forearm skin. Thereafter, the eligible participants were randomly assigned at a 1:1 ratio to either the experimental or control group and were blinded to the intervention assignment. The random assignment was performed by a third party, via the stratified permuted block randomization method. At the second visit, which was within a week after the first visit, the participants received either the active or sham device depending upon their randomized group assignment. They were instructed to wear the device for a total of 2 min, 1 min on each forearm every day for 4 weeks. One minute of UV exposure on each arm was controlled by the controller unit of the devices. Accordingly, the daily dose per unit area of UVB radiation by the active device was determined as approximately 7.8 mJ/cm2, and the effective area of exposure was ~80 cm2 for each forearm, making the total daily dose of 1.25 J (=7.8 mJ/cm2 × 80 cm2 × 2). For the safety of the participants, we monitored the occurrence of any adverse events and compliance with device use at the third and four visits, which took place 2 and 4 weeks after the initiation of the intervention, respectively. If any suspicious adverse skin reactions including erythema or rash occurred after the intervention, the use of the device was immediately discontinued, and the participant was withdrawn from the trial.
Serum 25-hydroxyvitamin D measurement
We adopted serum 25-hydroxyvitamin D [25(OH)D] concentrations as the indicator of serum vitamin D nutritional status since it is widely used for the measurement of serum vitamin D nutritional status [17]. Serum 25(OH)D concentrations were measured using a high-performance liquid-chromatography-tandem-mass-spectrometry method. Vitamin D is metabolized through the liver and kidneys and its metabolism is related to serum calcium and phosphate [18]. Therefore, the serum levels of calcium, phosphate, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total bilirubin, creatinine, and blood urea nitrogen were also assessed simultaneously with the serum 25(OH)D measurements. Blood tests were conducted four times, at the first (baseline), third (week 2), fourth (week 4), and final visits. The final visit was conducted two weeks after the end of the intervention (week 6). All the blood samples were collected during the daytime (from 9:30 A.M. to 4:00 P.M.), in the non-fasting state, and were properly processed and transported to the testing institute (Seoul Clinical Laboratories, Seoul, Republic of Korea). All the blood tests were performed at the same time of the day for each participant. In addition, the season during which the participants were enrolled in the study was considered as a confounding factor due to seasonal variation of serum 25(OH)D levels in Korean adult population [19]. Our study participants were categorized into four groups according to the season of study enrollment: spring (March to May), summer (June to August), autumn (September to November), and winter (December to February) group.
Demographic characteristics
Demographic information, including age, sex, body mass index, marital status, education level, current smoking status, drinking habits, and physical activity level, was obtained at the first visit. As for drinking habits, the participants were grouped as positive if they had consumed any alcohol at least once in the past month. With regard to the physical activity level, those who performed any kind of exercise for at least 30 min each, three times a week were classified as positive.
Nutrition and outdoor activities
Serum 25(OH)D levels can be affected by the skin production of vitamin D via sunlight exposure or dietary supplementation. Therefore, we examined the daily amount of dietary vitamin D intake and time spent on any outdoor activities at 2 and 4 weeks of intervention and 2 weeks after the end of the intervention. All participants were instructed to keep a daily diary of their diet and outdoor activity levels during the entire 4 weeks of the intervention period and the additional 2 weeks after the end of the intervention. We also calculated the average daily amount of dietary vitamin D intake and time spent on outdoor activities from the start of intervention to each assessment.
Statistical analysis
We calculated the minimal required sample size as 128 subjects (64 for each group), using G*Power software at a confidence level of 95%, an effect size of 0.5, and statistical power of 0.8. Effect size of 0.5 was adopted as it represents “medium” effect size. Considering the drop-out rate of 15%, we decided to enroll no fewer than 150 subjects (75 in each group). In the current study, a per-protocol (PP) analysis was performed after excluding the data from those who had dropped out.
Baseline demographic characteristics were compared using the independent t-test or Mann Whitney U test for continuous variables and the chi-squared test for categorical variables between the experimental and control groups. With regard to changes from baseline at each serum 25(OH)D level assessment, paired t-test and independent t-test (or Mann Whitney U test) were adopted to evaluate intra-group differences and inter-group differences, respectively. An analysis of covariance was also conducted to compare the changes in serum 25(OH)D levels after controlling for possible confounding factors. As a sub-analysis of the experimental group, we used independent t-test and ANCOVA to compare the changes of serum 25(OH)D levels after 4 weeks of intervention between those with and without vitamin D deficiency at the baseline assessment. Vitamin D deficiency was defined as serum 25(OH)D levels <20 ng/ml [20]. All statistical analyses were performed using SPSS version 25.0 for Windows (SPSS, Chicago, IL, USA) and a two-tailed p value of less than 0.05 was considered statistically significant.
Results
Eligibility and baseline demographic characteristics
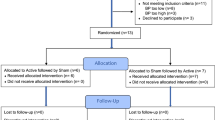
Among 160 healthy adults enrolled in the current study, one participant did not meet the inclusion criteria and three declined to participate. Six participants were excluded due to adverse skin reactions after using the test device at the screening visit. The remaining 150 participants were randomly assigned (1:1 ratio) to the experimental and control groups. During the study period, nine participants dropped out due to adverse skin reactions, and five were excluded for study protocol deviations. Finally, the data from 136 participants (64 in the experimental group and 72 in the control group) were included in the PP analysis (Fig. 2). Table 1 compares the baseline demographic factors between the experimental and control groups. There were no significant differences in the baseline demographic characteristics and season of study enrollment between the two study groups.
Safety and compliance
Of those who experienced adverse skin reactions during the study period, seven exhibited mild erythema without any other skin symptoms, which subsided spontaneously without treatment. The other two complained of mild erythema with swelling or itching and received topical treatment with ointment. All of these adverse reactions improved within 2 weeks after the onset of symptoms. In contrast, we did not find any adverse skin reaction in the control group, resulting in a significant between-group difference in adverse event rate after chi-square test (14.1% vs. 0%, p = 0.001).
Compliance with the device use was more than 95% in both groups at both assessment points after excluding those who dropped out due to adverse skin reactions. No meaningful between-group difference was found in compliance after 2 weeks of intervention (98.77% vs. 97.72%, Mann-Whitney U = 2166.5, p = 0.340). However, significantly higher compliance was observed in those with an active device after 4 weeks of intervention (97.88% vs. 95.59%, Mann-Whitney U = 1825.0, p = 0.017).
Nutrition and outdoor activities
Table 2 presents data regarding vitamin D nutrition and the outdoor activities of the study participants. No significant between-group difference was found in the average daily amount of dietary vitamin D intake and time in spent outdoor activities at each assessment.
Serum 25(OH)D levels
Table 3 describes the results of the serum 25(OH)D measurements. There was no significant difference in the baseline serum concentrations of 25(OH)D between the two groups. In the experimental group, although not statistically significant, a continuous, increasing trend was found in serum 25(OH)D levels. In contrast, the control group exhibited a significant decrease after 2 and 4 weeks of intervention. Of note, we observed a significant difference in changes in serum 25(OH)D levels between the two groups from baseline after 2 (0.25 ± 3.10 ng/mL vs. −1.07 ± 2.68 ng/mL, p = 0.009) and 4 weeks of intervention (0.75 ± 3.98 ng/ml vs. −1.75 ± 3.04 ng/ml, p < 0.001). This significant difference remained after adjusting for possible confounders such as age, sex, time spent on outdoor activities, dietary vitamin D intake, compliance with the device use, season of study enrollment, and baseline serum 25(OH)D levels. A follow-up assessment performed 2 weeks after the end of the intervention (week 6) revealed an additional increase in serum 25(OH)D levels in those with active devices, resulting in a significant between-group difference in the improvement of serum 25(OH)D status from baseline (0.99 ± 4.97 ng/mL vs. −1.70 ± 3.64 ng/mL, p = 0.001). Also, no significant between-group difference was found in other blood parameters that might be related to vitamin D metabolism (data not shown). After a sub-analysis of the experimental group, we found that those with vitamin D deficiency showed significantly higher increase of serum 25(OH) levels after 4 weeks of intervention, compared to those without vitamin D deficiency (2.18 ± 3.06 ng/mL vs. −0.78 ± 4.32 ng/mL, p = 0.002).
Discussion
The present study demonstrated that two minutes of the daily use of a proto-type wearable LED UVB light-emitting device for 4 weeks was effective in inducing the skin production of vitamin D in healthy adults. A significant difference was found in the change in serum 25(OH)D levels between the two groups after 2 and 4 weeks of intervention, supporting the efficacy of a proto-type wearable LED UVB device in improving serum 25(OH)D status.
A substantial decrease in serum 25(OH)D concentrations was observed in those with the sham device. In contrast, we found a trend toward increasing serum 25(OH)D levels in those with active devices, although it was not statistically significant. The possible explanation for this relatively small increase in serum 25(OH)D in the experimental group could be that the seasonal cycle of 25(OH)D status may have offset the effects of the active device on serum 25(OH)D status. In a large population-based study on serum 25(OH)D levels among healthy Korean adults, the authors observed the lowest and highest serum 25(OH)D levels in late winter (February) and early autumn (September), respectively [19]. Among our participants, 52 were enrolled during winter (from December to February) and 32 were enrolled during summer (from June to August). This seasonal difference in enrollment may have led to the declining tendency of serum 25(OH)D levels in the entire study cohort during the intervention period.
In addition, a decrease in outdoor activities due to the Coronavirus Disease 2019 pandemic may also have contributed to a further decline in serum 25(OH)D levels in both groups. In Korea, gatherings of many people were prohibited, and telecommuting was more popular than ever during the pandemic. These social distancing measures resulted in a significant increase in the time spent indoors. Notably, considering the reasons mentioned above, our data suggest that using a proto-type wearable LED UVB device could be helpful for the maintenance of adequate serum 25(OH)D status during periods of decreased serum 25(OH)D levels.
The most efficient UVB wavelength of LEDs for vitamin D production has not been clearly established yet. Several previous studies reported inconsistent results. A study by Hollick et al. found that the optimal wavelength range was between 293 and 298 nm [15]. In contrast, another study reported that a 285 nm LED was the most efficient in producing cutaneous vitamin D within five minutes of irradiation [21]. In this study, a 285 nm LED was chosen as the light source for the active devices due to its high efficiency within a short use time of fewer than five minutes.
In this study, the daily dose of UVB exposure by the active device was set as 15.6 mJ/cm2 for the safety of our participants. This dose was much less than the average minimal erythema dose, a minimal dose of UVB radiation that produces erythema in Koreans [22, 23]. We also performed UVB phototherapy on a relatively small skin area compared to other existing studies. For these reasons, although some adverse skin reactions such as mild erythema were observed in the experimental group, all of these adverse reactions were mild and reversible. Most of them were self-limited and only a few required additional treatment. The further improvement of proto-type wearable UVB devices may reduce the possibility of adverse effects, facilitating the clinical application of an advanced wearable device for vitamin D supplementation in the future.
Diet can be another source of vitamin D. However, oral preparations are ineffective for those with malabsorption syndromes [24]. Also, there are concerns about toxic effects such as confusion, vomiting, and abdominal pain by the excessive dietary intake of vitamin D [25]. In contrast, UVB phototherapy delivered via a wearable device has no risk of vitamin D intoxication since the human body can regulate the quantity of cutaneous vitamin D produced from UVB radiation [26].
The strengths of this study include a large study population consisting of healthy adults without medical and psychiatric comorbidities. In addition, we obtained information on the amount of sunlight exposure, dietary intake of vitamin D, and blood parameters related to vitamin D metabolism, all of which can affect serum 25(OH)D levels significantly. Moreover, our study was the first randomized controlled trial providing some evidence for the clinical application of a proto-type wearable LED UVB light-emitting device for vitamin D production in the general population.
This study also had several limitations to be considered. First, we performed PP analysis instead of intention-to-treat analysis, which may have led to selection bias. However, we found a low drop-out rate after excluding dropouts due to adverse skin reactions, and our data also found good compliance in both study groups, which could mitigate the possibility of selection bias. Second, the adverse skin reaction rate was 11.8% and this rate could be a considerable obstacle for clinical application of wearable devices for UVB phototherapy. However, all the adverse reactions had subsided without dermatological treatment. We also used the test device before intervention, to exclude participants with high risk of adverse skin reactions. In addition, we are developing a safer and more efficient device compared to the proto-type device used in this study. Third, we could not collect information on the Fitzpatrick skin type of each participant. Melanin pigment, the principal determinant of the Fitzpatrick skin type, might compromise vitamin D photosynthesis [27]. However, a recent study revealed that the inhibitory effects of melanin were limited [28]. Last, the study protocol was registered in the clinical trials registry after the initiation of the study. Although the CRIS, a Korean clinical trials registry platform, allows retrospective registration, we should have registered the study protocol in advance to meet international research standards.
In conclusion, the two-minute daily use of a proto-type wearable LED UVB device for 4 weeks was effective in improving serum 25(OH)D status. UVB phototherapy using a proto-type wearable device could be a novel and alternative option for vitamin D supplementation. Although we found only a modest increase of serum 25(OH)D, this study could be an introductory exploration for providing a possibility for clinical application of wearable devices. Furthermore, the clinical effects of a wearable device might be more remarkable when applied to a larger skin area over a longer period of time. Further research on the efficacy of wearable UVB LED devices of different wavelengths, such as 293 nm or 295 nm, is also needed to develop a more efficient wearable device. Given the considerable influence of inadequate vitamin D nutritional status on health, future longitudinal and methodologically robust studies are required to clarify the beneficial effects and safety of a wearable LED UVB device.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3:1535–41.
Holick MF, MacLaughlin J, Clark M, Holick S, Potts J, Anderson R, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5.
Engelsen O, Brustad M, Aksnes L, Lund E. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol. 2005;81:1287–90.
Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D: a preliminary study. Arch Dermatol. 1988;124:1802–4.
MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Investig. 1985;76:1536–8.
Pettifor JM, Moodley GP, Hough FS, Koch H, Chen T, Lu Z, et al. The effect of season and latitude on in vitro vitamin D formation by sunlight in South Africa. S Afr Med J. 1996;86:1270–2.
Malacova E, Cheang PR, Dunlop E, Sherriff JL, Lucas RM, Daly RM, et al. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of adults participating in the 2011–2013 Australian Health Survey. Br J Nutr. 2019;121:894–904.
Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Berisha AT, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–513.
Sallander E, Wester U, Bengtsson E, Wiegleb Edström D. Vitamin D levels after UVB radiation: effects by UVA additions in a randomized controlled trial. Photodermatol Photoimmunol Photomed. 2013;29:323–9.
Osmancevic A, Landin-Wilhelmsen K, Larkö O, Krogstad AL. Vitamin D status in psoriasis patients during different treatments with phototherapy. J Photochem Photobiol B. 2010;101:117–23.
Tangpricha V, Turner A, Spina C, Decastro S, Chen TC, Holick MF. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr. 2004;80:1645–9.
Pelaez EA.Villegas ER, LED power reduction trade-offs for ambulatory pulse oximetry. In: Proceedings of annual international conference on IEEE Engineering in Medicine and Biology Society. 2007;2007:2296–9.
Pulli T, Dönsberg T, Poikonen T, Manoocheri F, Kärhä P, Ikonen E. Advantages of white LED lamps and new detector technology in photometry. Light Sci Appl. 2015;4:e332.
Barnkob LL, Argyraki A, Petersen PM, Jakobsen J. Investigation of the effect of UV-LED exposure conditions on the production of vitamin D in pig skin. Food Chem. 2016;212:386–91.
Kalajian T, Aldoukhi A, Veronikis A, Persons K, Holick M. Ultraviolet b light emitting diodes (leds) are more efficient and effective in producing vitamin D 3 in human skin compared to natural sunlight. Sci Rep. 2017;7:1–8.
Veronikis AJ, Cevik MB, Allen RH, Shirvani A, Sun A, Persons KS, et al. Evaluation of a ultraviolet B light emitting diode (LED) for producing vitamin D3 in human skin. Anticancer Res. 2020;40:719–22.
Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017;8:947–57.
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–29.
Nah EH, Kim S, Cho H-I. Vitamin D levels and prevalence of vitamin D deficiency associated with sex, age, region, and season in Koreans. Lab Med Online. 2015;5:84–91.
Pfotenhauer KM, Shubrook JH. Vitamin D deficiency, its role in health and disease, and current supplementation recommendations. J Am Osteopath Assoc. 2017;117:301–5.
Ravichandran AK. Evaluation of a novel light-emitting diode device for producing vitamin D. (Boston University, 2015).
Park BS, Youn JI. Topographic measurement of skin color by narrow‐band reflectance spectrophotometer and minimal erythema dose (MED) in Koreans. Ski Res Technol. 1998;4:14–7.
Youn J, Oh J, Kim B, Suh D, Chung J, Oh S, et al. Relationship between skin phototype and MED in Korean, brown skin. Photodermatol Photoimmunol Photomed. 1997;13:208–11.
Lo C, Paris P, Clemens T, Nolan J, Holick M. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42:644–9.
Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D toxicity–a clinical perspective. Front Endocrinol. 2018:550.
Piotrowska A, Wierzbicka J, Żmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63:17–29.
Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67:1108–10.
Young AR, Morgan KA, Ho T-W, Ojimba N, Harrison GI, Lawrence KP, et al. Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: a comparison of extreme phenotypes. J Investig Dermatol. 2020;140:1418–26.e1.
Funding
This work was supported by the Engineering Research Center of Excellence (ERC) Program supported by the National Research Foundation (NRF), Korean Ministry of Science & ICT (MSIT) (Grant No. NRF-2017R1A5A1014708).
Author information
Authors and Affiliations
Contributions
HJL, SY, TK, JP, and I-YY: designed the research; JKH, JSA, and EL conducted the research study; SY, HM, SK, and JP provided essential research materials; HJL, JP, and I-YY: performed statistical analyses and wrote the manuscript; HJL, SY, JP, and I-YY critically reviewed the manuscript; I-YY had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H.J., Yoo, S., Hong, J.K. et al. The effect of proto-type wearable light-emitting devices on serum 25-hydroxyvitamin D levels in healthy adults: a 4-week randomized controlled trial. Eur J Clin Nutr 77, 342–347 (2023). https://doi.org/10.1038/s41430-022-01241-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01241-z
This article is cited by
-
UVB-emitting cloth to prevent low serum 25-hydroxyvitamin D caused by clothing
European Journal of Clinical Nutrition (2023)