Abstract
Background
Interindividual variations in body mass index (BMI) can be partially explained by genetic differences. We aimed to examine the association of the ADIPOQ-rs2241766, LEP-rs7799039 and FTO-rs9939609 genetic variants with BMI trajectory in women of reproductive age over 6 years of follow-up.
Methods
This was a prospective study that used data from 435 women of the PREDI Study conducted in Brazil. Socioeconomic, biological and anthropometric data were collected at four time points: 2012 (baseline) in the maternity hospital, and 2013–14, 2016–17 and 2018 (1st, 2nd and 3rd follow-ups) at the participant’s home. Genotyping was performed by PCR-RFLP. Linear mixed-effect and Poisson regression models were used to address the association of ADIPOQ, LEP and FTO genotypes with BMI and overweight/obesity status.
Results
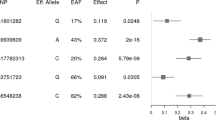
Women carrying the risk allele (TA or AA) of the FTO-rs9939609 genetic variant had a 1.16 kg/m2 higher BMI over the follow-up period than those carrying the wild-type genotype (TT), even when adjusted for potential confounders (95% CI: 0.23–2.10, p = 0.015). The risk of obesity associated with the FTO-TA or AA genotype decreased over the years, demonstrating an influence of time on its trajectory (IRR = 0.99, 95% CI: 0.98–0.99, p = 0.016). There was no variation in BMI trajectories for the ADIPOQ-rs2241766, LEP-rs7799039 or FTO-rs9939609 genetic variant.
Conclusions
The results of this study suggest that monitoring women of reproductive age with ADIPOQ-rs2241766 TG/GG or FTO-rs9939609 TA/AA genotypes may be an important strategy to reduce maternal excess body weight and, consequently, the long-term public health burden of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
WHO—World Health Organization. Obesity. Preventing and managing the global epidemic. Geneve; 2000.
WHO—World Health Organization. Obesity and overweight. 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Hu FB. Overweight and obesity in women: health risks and consequences. J Women’s Health. 2003;12:163–72.
Templeton A. Obesity and women’s health. Facts Views Vis Obgyn. 2014;6:175–6.
Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nature reviews. Dis Prim. 2017;3:17034.
Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol. 2012;3:29.
Triantafyllou GA, Paschou SA, Mantzoros CS. Leptin and hormones: energy homeostasis. Endocrinol Metab Clin North Am. 2016;45:633–45.
Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014;15:149–56.
Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23:770–84.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83.
Heid IM, Wagner SA, Gohlke H, Iglseder B, Mueller JC, Cip P, et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes 2006;55:375–84.
Pollin TI, Tanner K, O’Connell JR, Ott SH, Damcott CM, Shuldiner AR, et al. Linkage of plasma adiponectin levels to 3q27 explained by association with variation in the APM1 gene. Diabetes 2005;54:268–74.
Kroll C, Mastroeni SSBS, Veugelers PJ, Mastroeni MF. Associations of ADIPOQ and LEP gene variants with energy intake: a systematic review. Nutrients 2019;11:750.
Boumaiza I, Omezzine A, Rejeb J, Rebhi L, Ouedrani A, Ben Rejeb N, et al. Relationship between leptin G2548A and leptin receptor Q223R gene polymorphisms and obesity and metabolic syndrome risk in Tunisian volunteers. Genet Test Mol Biomark. 2012;16:726–33.
Feng H, Zheng L, Feng Z, Zhao Y, Zhang N. The role of leptin in obesity and the potential for leptin replacement therapy. Endocrine 2013;44:33–9.
Friedman JM. Leptin and the regulation of body weigh. Keio J Med. 2011;60:1–9.
Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–88.
Mizuno TM. Fat Mass and Obesity Associated (FTO) Gene and Hepatic Glucose and Lipid Metabolism. Nutrients. 2018;10:1–11.
Speakman JR. The ‘Fat Mass and Obesity Related’ (FTO) gene: mechanisms of impact on obesity and energy balance. Curr Obes Rep. 2015;4:73–91.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94.
Doaei S, Mosavi Jarrahi SA, Sanjari Moghadam A, Akbari ME, Javadi Kooshesh S, Badeli M, et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis. Biomol Concepts. 2019;10:237–42.
Karra E, O’Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Investig. 2013;123:3539–51.
IBGE. Brazilian Institute of Geography and Statistics. Joinville, 2017. https://cidades.ibge.gov.br/brasil/sc/joinville/panorama.
Kroll C, Mastroeni SS, Veugelers PJ, Mastroeni MF. Association of ADIPOQ, LEP, and FTO gene polymorphisms with large for gestational age infants. Am J Hum Biol. 2017;29:1002.
Mastroeni MF, Czarnobay SA, Kroll C, Figueiredo KB, Mastroeni SS, Silva JC, et al. The independent importance of pre-pregnancy weight and gestational weight gain for the prevention of large-for gestational age Brazilian newborns. Matern Child Health J. 2016;21:705–14.
Mastroeni MF, Mastroeni S, Czarnobay SA, Ekwaru JP, Loehr SA, Veugelers PJ. Breast-feeding duration for the prevention of excess body weight of mother-child pairs concurrently: a 2-year cohort study. Public Health Nutr. 2017;20:2537–48.
Kroll C, de Franca PHC, Mastroeni MF. Association between FTO gene polymorphism and excess body weight in women from before to after pregnancy: a cohort study. Am J Hum Biol: Off J Hum Biol Counc. 2018;30:e23164.
Gordon C, Chumlea W, Roche A. Stature, recumbent length, and weight. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization: reference manual. Champaign: Human Kinetics; 1988. p. 3–8.
Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Orientações para a coleta e análise de dados antropométricos em serviços de saúde: Norma Técnica do Sistema de Vigilância Alimentar e Nutricional - SISVAN/Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica. Brasília: Ministério da Saúde; 2011.
Kline MC, Duewer DL, Redman JW, Butler JM, Boyer DA. Polymerase chain reaction amplification of DNA from aged blood stains: quantitative evaluation of the “suitability for purpose” of four filter papers as archival media. Anal Chem. 2002;74:1863–9.
Low CF, Mohd Tohit ER, Chong PP, Idris F. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet. 2011;283:1255–60.
Hinuy HM, Hirata MH, Forti N, Diament J, Sampaio MF, Armaganijan D, et al. Leptin G-2548A promoter polymorphism is associated with increased plasma leptin and BMI in Brazilian women. Arch Endocrinol Metab. 2008;52:611–6.
Lopez-Bermejo A, Petry CJ, Diaz M, Sebastiani G, de Zegher F, Dunger DB, et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab. 2008;93:1501–5.
Hinuy HM. Avaliação de regiões hipervariáveis de genes que predispõem à obesidade. Dissertation. São Paulo: School of Pharmaceutical Sciences, University of São Paulo; 2004.
Sales WB, Silleno JD,Jr, Kroll C, Mastroeni SSBS, Silva JC, Mastroeni MF.Influence of altered maternal lipid profile on the lipid profile of the newborn. Arch Endocrinol Metab. 2015;59:123–8.
NCBI—National Center for Biotechnology Information. 1000 Genomes Browser. 2020.
de Oliveira R, Moraes TI, Cerda A, Hirata MH, Fajardo CM, Sousa MC, et al. ADIPOQ and IL6 variants are associated with a pro-inflammatory status in obeses with cardiometabolic dysfunction. Diabetol Metab Syndr. 2015;7:34.
Martins MC, Trujillo J, Freitas-Vilela AA, Farias DR, Rosado EL, Struchiner CJ, et al. Associations between obesity candidate gene polymorphisms (fat mass and obesity-associated (FTO), melanocortin-4 receptor (MC4R), leptin (LEP) and leptin receptor (LEPR)) and dietary intake in pregnant women. Br J Nutr. 2018;120:454–63.
Melistas L, Mantzoros CS, Kontogianni M, Antonopoulou S, Ordovas JM, Yiannakouris N. Association of the +45T>G and +276G>T polymorphisms in the adiponectin gene with insulin resistance in nondiabetic Greek women. Eur J Endocrinol. 2009;161:845–52.
do Nascimento GA, Leite N, Furtado-Alle L, Teixeira MD, de Souza RLR, Milano GE, et al. FTO rs9939609 does not interact with physical exercise but influences basal insulin metabolism in Brazilian overweight and obese adolescents. J Obes. 2018;2018:3134026.
Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet. 2010;18:1054–6.
Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–72.
Steemburgo T, Azevedo MJ, Gross JL, Milagro FI, Campion J, Martinez JA. The rs9939609 polymorphism in the FTO gene is associated with fat and fiber intakes in patients with type 2 diabetes. J Nutrigenetics Nutrigenomics. 2013;6:97–106.
den Hoed M, Westerterp-Plantenga MS, Bouwman FG, Mariman EC, Westerterp KR. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am J Clin Nutr. 2009;90:1426–32.
Emond JA, Tovar A, Li Z, Lansigan RK, Gilbert-Diamond D. FTO genotype and weight status among preadolescents: assessing the mediating effects of obesogenic appetitive traits. Appetite 2017;117:321–9.
Sonestedt E, Gullberg B, Ericson U, Wirfalt E, Hedblad B, Orho-Melander M. Association between fat intake, physical activity and mortality depending on genetic variation in FTO. Int J Obes. 2011;35:1041–9.
Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3.
Labayen I, Ruiz JR, Ortega FB, Dallongeville J, Jimenez-Pavon D, Castillo MJ, et al. Association between the FTO rs9939609 polymorphism and leptin in European adolescents: a possible link with energy balance control. The HELENA study. Int J Obes. 2011;35:66–71.
Wang P, Yang FJ, Du H, Guan YF, Xu TY, Xu XW, et al. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol Med. 2011;17:523–32.
Rask-Andersen M, Almen MS, Olausen HR, Olszewski PK, Eriksson J, Chavan RA, et al. Functional coupling analysis suggests link between the obesity gene FTO and the BDNF-NTRK2 signaling pathway. BMC Neurosci. 2011;12:117.
Lin L, Hales CM, Garber K, Jin P. Fat mass and obesity-associated (FTO) protein interacts with CaMKII and modulates the activity of CREB signaling pathway. Hum Mol Genet. 2014;23:3299–306.
Celis-Morales C, Marsaux CF, Livingstone KM, Navas-Carretero S, San-Cristobal R, O’Donovan CB, et al. Physical activity attenuates the effect of the FTO genotype on obesity traits in European adults: the Food4Me study. Obesity. 2016;24:962–9.
Li H, Kilpelainen TO, Liu C, Zhu J, Liu Y, Hu C, et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 2012;55:981–95.
Yang WS, Tsou PL, Lee WJ, Tseng DL, Chen CL, Peng CC, et al. Allele-specific differential expression of a common adiponectin gene polymorphism related to obesity. J Mol Med. 2003;81:428–34.
Lu JF, Zhou Y, Huang GH, Jiang HX, Hu BL, Qin SY. Association of ADIPOQ polymorphisms with obesity risk: a meta-analysis. Hum Immunol. 2014;75:1062–8.
Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJG. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–71.
Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9.
Zayani N, Omezzine A, Boumaiza I, Achour O, Rebhi L, Rejeb J, et al. Association of ADIPOQ, leptin, LEPR, and resistin polymorphisms with obesityparameters in Hammam Sousse Sahloul Heart Study. J Clin Lab Anal. 2017;31:1–10.
Zhang L, Yuan LH, Xiao Y, Lu MY, Zhang LJ, Wang Y. Association of leptin gene -2548 G/A polymorphism with obesity: a meta-analysis. Ann Nutr Metab. 2014;64:127–36.
Constantin A, Costache G, Sima AV, Glavce CS, Vladica M, Popov DL. Leptin G-2548A and leptin receptor Q223R gene polymorphisms are not associated with obesity in Romanian subjects. Biochem Biophys Res Commun. 2010;391:282–6.
Yan J, Wang X, Tao H, Yang W, Luo M, Lin F. Lack of association between leptin G-2548A polymorphisms and obesity risk: evidence based on a meta-analysis. Obes Res Clin Pract. 2015;9:389–97.
Nicolaidis S. Environment and obesity. Metab: Clin Exp. 2019;100s:153942.
Corrêa CB, Mastroeni S, Mastroeni MF. Effect of age at menarche on the mother’s weight status two and four years after delivery: a cohort study. Women Health. 2020;60:1196–205.
Iversen DS, Kesmodel US, Ovesen PG. Associations between parity and maternal BMI in a population-based cohort study. Acta Obstetricia et Gynecologica Scandinavica 2018;97:694–700.
Labayen I, Ruiz JR, Huybrechts I, Ortega FB, Arenaza L, Gonzalez-Gross M, et al. Dietary fat intake modifies the influence of the FTO rs9939609 polymorphism on adiposity in adolescents: the HELENA cross-sectional study. Nutr Metab Cardiovasc Dis. 2016;26:937–43.
Qi Q, Downer MK, Kilpeläinen TO, Taal HR, Barton SJ, Ntalla I, et al. Dietary intake, FTO genetic variants, and adiposity: a combined analysis of over 16,000 children and adolescents. Diabetes 2015;64:2467–76.
Bjornland T, Langaas M, Grill V, Mostad IL. Assessing gene-environment interaction effects of FTO, MC4R and lifestyle factors on obesity using an extreme phenotype sampling design: results from the HUNT study. PloS ONE. 2017;12:e0175071.
Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8:e1001116.
SHAd SilvaJunior, Santos SM, Coeli CM, Carvalho MS. Assessment of participation bias in cohort studies: systematic review and meta-regression analysis. Cad de Saúde Pública. 2015;31:2259–74.
Shin D, Chung H, Weatherspoon L, Song WO. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J. 2014;18:1667–74.
Lundblad MW, Jacobsen BK. The reproducibility of self-reported age at menarche: the Tromsø Study. BMC Women’s Health. 2017;17:62.
Acknowledgements
We thank the Darcy Vargas Maternity Hospital and the Gimenes Laboratory of Joinville, Santa Catarina, Brazil, for permitting the data collection at their facilities; the University of Joinville Region, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Funding
This research was supported in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, through a scholarship granted to Caroline Kroll (Grant Number 88887.149709/2017-00), and by the Fundo de Apoio à Pesquisa/University of Joinville Region—FAP/UNIVILLE Brazil (Grant Numbers 4555/2011, 01/2014 and 02/2016).
Author information
Authors and Affiliations
Contributions
CK formulated the research question, collected and analysed the data and wrote the manuscript. DRF analysed the data and revised the manuscript. TRBC analysed the data and revised the manuscript. GK suggested analyses and critically revised the manuscript. MFM organized and designed the study, formulated the research question and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was approved by the Research Ethics Committee of UNIVILLE (Protocol No. 107/2011). All participants gave their informed consent before inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kroll, C., Farias, D.R., Carrilho, T.R.B. et al. Association of ADIPOQ-rs2241766 and FTO-rs9939609 genetic variants with body mass index trajectory in women of reproductive age over 6 years of follow-up: the PREDI study. Eur J Clin Nutr 76, 159–172 (2022). https://doi.org/10.1038/s41430-021-00911-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-021-00911-8