Abstract
Background
Recent studies have reported mixed results on the association between the pro-inflammatory dietary index and risk of breast cancer. We perform this comprehensive meta-analysis to figure out whether high dietary inflammatory index (DII) score is a risk factor for the occurrence of breast cancer.
Methods
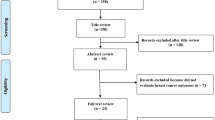
We comprehensively searched the PubMed, EMBASE and Cochrane databases to identify included studies updated to September 12, 2017. All studies that reported risk estimates by comparing the highest DII score to the lowest were assessed.
Results
A total of seven observational studies were identified: three case controls and four cohorts, involving 319,993 participants. Overall, the meta-analysis reported that individuals with the highest DII score were associated with a 25% increased risk of breast cancer versus those with the lowest DII score (relative risk [RR] = 1.25; 95% confidence interval [CI] 1.09–1.44; I2 = 82.7%, p = 0.000). Upon stratified analysis, significant positive associations remained for postmenopausal women (RR = 1.15; 95% CI 1.02–1.30; p = 0.020), case-control studies (RR = 1.68; 95% CI 1.13–2.49; p = 0.010), Asia (RR = 2.30; 95% CI 1.7–3.12; p = 0.0031) and Europe (RR = 1.26; 95% CI 1.01–1.58; p = 0.0477). When analysed on hormonal receptor status, 36% increased risk was explored for hormone-receptor negative.
Conclusion
This meta-analysis suggested that more pro-inflammatory diets (higher DII scores) are associated with increased breast cancer incidence. However, the research is not about significant associations but about moderate effect sizes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. https://doi.org/10.3322/caac.21262
Touvier M. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177:3–13. https://doi.org/10.1093/aje/kws359
Rogers AB, Houghton J. Inflammation and cancer. Nature. 2009;420:860–7.
Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35:1–16. https://doi.org/10.1089/jir.2014.0026
Agnoli C, Grioni S, Pala V, Allione A, Matullo G, Gaetano CD, et al. Biomarkers of inflammation and breast cancer risk: a case-control study nested in the EPIC-Varese cohort. Sci Rep. 2017;7:12708. https://doi.org/10.1038/s41598-017-12703-x
Chan DS, Bandera EV, Greenwood DC, Norat T. Circulating C-Reactive Protein and Breast Cancer Risk-Systematic Literature Review and Meta-analysis of Prospective Cohort Studies. Cancer Epidemiol, Biomark Prev. 2015;24:1439–49. https://doi.org/10.1158/1055-9965.EPI-15-0324
Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–24. https://doi.org/10.1200/JCO.2008.19.8440
Ricordi C, Garcia-Contreras M. Farnetti S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J Am Coll Nutr. 2015;34(Suppl 1):10–3. https://doi.org/10.1080/07315724.2015.1080101
Potentas E, Witkowska AM, Zujko ME. Mediterranean diet for breast cancer prevention and treatment in postmenopausal women. Prz Menopauzalny. 2015;14:247–53. https://doi.org/10.5114/pm.2015.56381
Van Den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer. 2017;140:2220–31. https://doi.org/10.1002/ijc.30654
Pot GK, Stephen AM, Dahm CC, Key TJ, Cairns BJ, Burley VJ, et al. Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK Dietary Cohort Consortium. Eur J Clin Nutr. 2014;68:1353–8. https://doi.org/10.1038/ejcn.2014.135
Mullie P, Koechlin A, Boniol M, Autier P, Boyle P. Relation between Breast Cancer and High Glycemic Index or Glycemic Load: A Meta-analysis of Prospective Cohort Studies. Crit Rev Food Sci Nutr. 2016;56:152–9. https://doi.org/10.1080/10408398.2012.718723
Makarem N, Chandran U, Bandera EV, Parekh N. Dietary fat in breast cancer survival. Annu Rev Nutr. 2013;33:319–8. https://doi.org/10.1146/annurev-nutr-112912-095300
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. https://doi.org/10.1017/S1368980013002115
Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–72. https://doi.org/10.3945/jn.109.114025
Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients. 2017;9:E1043 https://doi.org/10.3390/nu9091043
Zhong X, Guo L, Zhang L, Li Y, He R, Cheng G. Inflammatory potential of diet and risk of cardiovascular disease or mortality: A meta-analysis. Sci Rep. 2017;7:6367 https://doi.org/10.1038/s41598-017-06455-x
Fan Y, Jin X, Man C, Gao Z, Wang X. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget. 2017;8:59592–600. https://doi.org/10.18632/oncotarget.19233
Ge I, Rudolph A, Shivappa N, Flesch-Janys D, Hebert JR, Chang-Claude J. Dietary inflammation potential and postmenopausal breast cancer risk in a German case-control study. Breast. 2015;24:491–6. https://doi.org/10.1016/j.breast.2015.04.012
Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Johnson KC, et al. Patterns of change over time and history of the inflammatory potential of diet and risk of breast cancer among postmenopausal women. Breast Cancer Res Treat. 2016;159:139–49. https://doi.org/10.1007/s10549-016-3925-6
Huang WQ, Mo XF, Ye YB, Shivappa N, Lin FY, Huang J, et al. A higher Dietary Inflammatory Index score is associated with a higher risk of breast cancer among Chinese women: a case-control study. Br J Nutr. 2017;117:1358–67. https://doi.org/10.1017/S0007114517001192
Shivappa N, Hebert JR, Rosato V, Montella M, Serraino D, La Vecchia C. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. Mol Nutr Food Res. 2017;61. https://doi.org/10.1002/mnfr.201600500.
Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hebert JR. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. 2017;61. https://doi.org/10.1002/mnfr.201600592.
Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Nutr. 2016;114:1277–85. https://doi.org/10.1038/bjc.2016.98
Shivappa N, Sandin S, Lof M, Hebert JR, Adami HO, Weiderpass E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Nutr. 2015;113:1099–103. https://doi.org/10.1038/bjc.2015.304
Wells GA, Shea B, O'connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale NOS for assessing the quality of nonrandomized studies in meta-analysis. Appl Eng Agric. 2014;18:727–34.
Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30.
Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126–51. https://doi.org/10.1177/016327870102400203
Fung TT, Hu FB, Mccullough ML, Newby PK, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136:466–72.
Buckland G, Travier N, Cottet V, Gonzalez CA, Lujan-Barroso L, Agudo A, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer. 2013;132:2918–27. https://doi.org/10.1002/ijc.27958
Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. 2017;75:405–19. https://doi.org/10.1093/nutrit/nux012
Castelló A, Boldo E, Pérez-Gómez B, Lope V, Altzibar JM, Martín V, et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas. 2017;103:8–15. https://doi.org/10.1016/j.maturitas.2017.06.020
Phi XA, Houssami N, Hooning MJ, Riedl CC, Leach MO, Sardanelli F, et al. Accuracy of screening women at familial risk of breast cancer without a known gene mutation: Individual patient data meta-analysis. Eur J Cancer. 2017;85:31–8. https://doi.org/10.1016/j.ejca.2017.07.055
Boodram JN, Mcgregor IJ, Bruno PM, Cressey PB, Hemann MT, Suntharalingam K. Breast Cancer Stem Cell Potent Copper(II)-Non-Steroidal Anti-Inflammatory Drug Complexes. Angew Chem Int Ed. 2016;55:2845–50. https://doi.org/10.1002/anie.201510443
Padamsee TJ, Wills CE, Yee LD, Paskett ED. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res. 2017;19:34. https://doi.org/10.1186/s13058-017-0826-5
Lagiou A, Lagiou P. Tobacco smoking and breast cancer: a life course approach. Eur J Epidemiol. 2017. https://doi.org/10.1007/s10654-017-0300-9
Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56:986–9. https://doi.org/10.1097/JOM.0000000000000213
Wirth MD, Hebert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, et al. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res. 2016;36:214–9. https://doi.org/10.1016/j.nutres.2015.11.009
Julia C, Assmann KE, Shivappa N, Hebert JR, Wirth MD, Hercberg S, et al. Long-term associations between inflammatory dietary scores in relation to long-term C-reactive protein status measured 12 years later: findings from the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) cohort. Br J Nutr. 2017;117:306–14. https://doi.org/10.1017/S0007114517000034
Wirth MD, Shivappa N, Davis L, Hurley TG, Ortaglia A, Drayton R, et al. Construct Validation of the Dietary Inflammatory Index among African Americans. J Nutr Health Aging. 2017;21:487–91. https://doi.org/10.1007/s12603-016-0775-1
Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy. 2015;45:177–83. https://doi.org/10.1111/cea.12323
Spennati GF, Placidi S, Persichetti B, De Matteis F. Renin and aldosterone response in human newborns to acute blood volume change. Arch Dis Child. 1979;54:80.
Thompson FE, Metzner HL, Lamphiear DE, Hawthorne VM. Characteristics of individuals and long term reproducibility of dietary reports: the Tecumseh Diet Methodology Study. J Clin Epidemiol. 1990;43:1169–78.
Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, et al. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the coronary artery risk development in young adults study. Am J Clin Nutr. 2012;95:580–6. https://doi.org/10.3945/ajcn.111.020719
Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 81473513).
Author contributions
LW and CL contributed equally to this paper and are co-first authors. LW, CL and CS designed the research; FF, LL and TZ searched databases and collected full-text papers; LW, CL and CZ interpreted and extracted the data; LW, CL and LZ wrote the manuscript; CS and JT secured funds, supervised the study and made critical revision of the manuscript. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These author contributed equally: Lu Wang, Cun Liu.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, L., Liu, C., Zhou, C. et al. Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. Eur J Clin Nutr 73, 509–517 (2019). https://doi.org/10.1038/s41430-018-0196-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-018-0196-9
This article is cited by
-
Dietary Inflammatory Index and risk of breast cancer: evidence from a prospective cohort of 67,879 women followed for 20 years in France
European Journal of Nutrition (2023)
-
Dietary inflammatory index and breast cancer risk: an updated meta-analysis of observational studies
European Journal of Clinical Nutrition (2022)
-
A case–control study in France showing that a pro-inflammatory diet is associated with a higher risk of breast cancer
Scientific Reports (2021)
-
Folate intake and the risk of breast cancer: an up-to-date meta-analysis of prospective studies
European Journal of Clinical Nutrition (2019)