Abstract
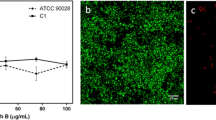
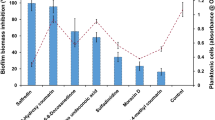
Multispecies biofilms, in which both fungus and bacteria species can be present, play a significant role in persistent infections, and new therapeutic options are needed against them. In this study, the activities of ceragenins and antimicrobial peptides (AMPs) (magainin, cecropin A, LL-37) were investigated against multispecies biofilms formed by Candida albicans and four clinically important Gram-negative bacteria, including Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and Klebsiella pneumoniae. Our results show that CSA-13 and CSA-90 were the most effective agents against both mono and multispecies biofilms (P < 0.05). CSA-131 and CSA-192 showed the least antimicrobial activity against mono and fungal-bacterial multispecies biofilms. Inhibition of multispecies biofilms with CSA-13 and CSA-90 was also confirmed through fluorescence microscopy images. When AMPs evaluated alone, they proved ineffective against both C. albicans and Gram-negative bacteria at the concentrations tested. In these studies, ceragenins were much more effective than AMPs against multi or monospecies biofilms, especially those containing C. albicans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, Williams C, Munro CA, Jones BJ, Ramage G. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin Microbiol Infect. 2016;22:87–93.
Arzmi MH, Dashper S, McCullough M. Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J Oral Pathol Med. 2019;48:546–51.
Esher SK, Fidel PL, Noverr MC. Candida/Staphylococcal polymicrobial intra-abdominal infection: pathogenesis and perspectives for a novel form of trained innate immunity. J Fungi. 2019;5:37.
Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18:310–21.
Lopes SP, Azevedo NF, Pereira MO. Microbiome in cystic fibrosis: shaping polymicrobial interactions for advances in antibiotic therapy. Crit Rev Microbiol. 2015;41:353–65.
Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annu Rev Microbiol. 2015;69:71–92.
Rodrigues ME, Lopes SP, Pereira CR, Azevedo NF, Lourenço A, Henriques M, Pereira MO. Polymicrobial ventilator-associated pneumonia: fighting in vitro Candida albicans-Pseudomonas aeruginosa biofilms with antifungal-antibacterial combination therapy. PLoS ONE. 2017;12:e0170433.
Harriott MM, Noverr MC. Importance of Candida–bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19:557–63.
Fourie R, Pohl CH. Beyond antagonism: the interaction between Candida species and Pseudomonas aeruginosa. J Fungi. 2019;5:34.
Hirota K, Yumoto H, Sapaar B, Matsuo T, Ichikawa T, Miyake Y. Pathogenic factors in Candida biofilm‐related infectious diseases. J Appl Microbiol. 2017;122:321–30.
Peleg AY, Tampakakis E, Fuchs BB, Eliopoulos GM, Moellering RC Jr, Mylonakis E. Prokaryote–eukaryote interactions identified by using Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:14585–90.
El‐Azizi MA, Starks SE, Khardori N. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Appl Microbiol. 2004;96:1067–73.
Hager CL, Isham N, Schrom KP, Chandra J, McCormick T, Miyagi M, Ghannoum MA. Effects of a novel probiotic combination on pathogenic bacterial-fungal polymicrobial biofilms. mBio. 2019;10:e00338–19.
Vuotto C, Longo F, Balice M, Donelli G, Varaldo P. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens. 2014;3:743–58.
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15:740.
Ghosh S, Joseph G, Korza G, He L, Yuan JH, Dong W, Setlow B, Li YQ, Savage PB, Setlow P. Effects of the microbicide ceragenin CSA‐13 on and properties of Bacillus subtilis spores prepared on two very different media. J Appl Microbiol. 2019;127:109–20.
Hacioğlu M, Hacıosmanoğlu E, Birteksöz Tan AS, Bozkurt Guzel Ç, Savage P. Effects of ceragenins and conventional antimicrobials on Candida albicans and Staphylococcus aureus mono and multispecies biofilms. Diagn Microbiol Infect Dis. 2019;95:114863. https://doi.org/10.1016/j.diagmicrobio.2019.06.014.
Hashemi M, Holden B, Taylor M, Wilson J, Coburn J, Hilton B, Nance T, Gubler S, Genberg C, Deng S, Savage PB. Antibacterial and antifungal activities of poloxamer micelles containing ceragenin CSA-131 on ciliated tissues. Molecules. 2018;23:596.
Hashemi M, Mmuoegbulam A, Holden B, Coburn J, Wilson J, Taylor MF, Reiley J, Baradaran D, Stenquist T, Deng S, Savage PB. Susceptibility of multidrug-resistant bacteria, isolated from water and plants in Nigeria, to Ceragenins. Int J Environ Res Public Health. 2018;15:2758.
Wnorowska U, Piktel E, Durnaś B, Fiedoruk K, Savage PB, Bucki R. Use of ceragenins as a potential treatment for urinary tract infections. BMC Infect Dis. 2019;19:369.
Durnaś B, Wnorowska U, Pogoda K, Deptuła P, Wątek M, Piktel E, et al. Candidacidal activity of selected ceragenins and human cathelicidin LL-37 in experimental settings mimicking infection sites. PLoS ONE. 2016;11:e0157242.
Lai XZ, Feng Y, Pollard J, Chin JN, Rybak MJ, Bucki R, et al. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res. 2008;41:1233–40.
Peters BM, Ward RM, Rane HS, Lee SA, Noverr MC. Efficacy of ethanol against Candida albicans and Staphylococcus aureus polymicrobial biofilms. Antimicrob Agents Chemother. 2013;57:74–82.
Nagant C, Pitts B, Stewart PS, Feng Y, Savage PB, Dehaye JP. Study of the effect of antimicrobial peptide mimic, CSA-13, on an established biofilm formed by Pseudomonas aeruginosa. Microbiologyopen. 2013;2:318–25.
Tavernier S, Crabbé A, Hacioglu M, Stuer L, Henry S, Rigole P, et al. Community composition determines activity of antibiotics against multispecies biofilms. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/AAC.00302-17.
Li CS, Chia WC, Chen PS. Fluorochrome and flow cytometry to monitor microorganisms in treated hospital wastewater. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42:195–203.
Bektaş M, Hacıosmanoğlu E, Ozerman B, Varol B, Nurten R, Bermek E. On diphtheria toxin fragment A release into the cytosol–cytochalasin D effect and involvement of actin filaments and eukaryotic elongation factor 2. Int J Biochem Cell Biol. 2011;43:1365–72. https://doi.org/10.1016/j.biocel.2011.05.017.
Bozkurt-Guzel C, Hacioglu M, Savage PB. Investigation of the in vitro antifungal and antibiofilm activities of ceragenins CSA-8, CSA-13, CSA-44, CSA-131, and CSA-138 against Candida species. Diagn Microbiol Infect Dis. 2018a;91:324–30. https://doi.org/10.1016/j.diagmicrobio.2018.03.014.
Moscoso M, Esteban-Torres M, Menéndez M, García E. In vitro bactericidal and bacteriolytic activity of ceragenin CSA-13 against planktonic cultures and biofilms of Streptococcus pneumoniae and other pathogenic streptococci. PLoS ONE. 2014;9:e101037. https://doi.org/10.1371/journal.pone.0101037.
Leszczynska K, Namiot D, Byfield FJ. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J Antimicrob Chemother. 2013;68:610–18.
Olekson MA, You T, Savage PB, Leung KP. Antimicrobial ceragenins inhibit biofilms and affect mammalian cell viability and migration in vitro. FEBS Open Bio. 2017. https://doi.org/10.1002/2211-5463.12235.
Bozkurt-Guzel C, Oyardi O, B Savage P. Comparative in vitro antimicrobial activities of CSA-142 and CSA-192, second-generation ceragenins, with CSA-13 against various microorganisms. J Chemother. 2018b;30:332–7. https://doi.org/10.1080/1120009X.2018.1534567.
Geitani R, Ayoub Moubareck C, Touqui L, Karam Sarkis D. Cationic antimicrobial peptides: alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol. 2019;19:54. https://doi.org/10.1186/s12866-019-1416-8.
Dosler S, Karaaslan E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides. 2014;62:32–7. https://doi.org/10.1016/j.peptides.2014.09.021.
Acknowledgements
This work was supported by the Research Fund of Istanbul University [project number TSA-2017-26191].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, M., Oyardi, O., Bozkurt-Guzel, C. et al. Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J Antibiot 73, 455–462 (2020). https://doi.org/10.1038/s41429-020-0299-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0299-0
This article is cited by
-
Antifungal properties of cathelicidin LL-37: current knowledge and future research directions
World Journal of Microbiology and Biotechnology (2024)
-
Antibiofilm properties of cathelicidin LL-37: an in-depth review
World Journal of Microbiology and Biotechnology (2023)
-
Effects of Antimicrobial Peptides Gal-13 on the Growth Performance, Intestinal Microbiota, Digestive Enzyme Activities, Intestinal Morphology, Antioxidative Activities, and Immunity of Broilers
Probiotics and Antimicrobial Proteins (2023)
-
Combating polymicrobial biofilm: recent approaches
Folia Microbiologica (2023)