Abstract
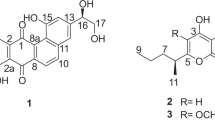
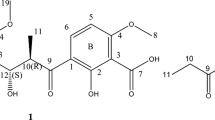
Fungi are important resources for drug development, as they have a diversity of genes, that can produce novel secondary metabolites with effective bioactivities. Here, five depsidone-based analogs were isolated from the rice media of Chaetomium brasiliense SD-596. Their structures were elucidated using NMR and mass spectrometry analysis. Five compounds, including three new depsidone analogs, mollicellin S (1), mollicellin T (2), and mollicellin U (3), and two known compounds, mollicellin D (4) and mollicellin H (5), exhibited significant inhibition against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA), with MIC values ranging from 6.25 to 12.5 μg ml−1. Herein, we identified the predicted plausible biosynthetic cluster of the compounds and discussed the structure-activity relationship. Finally, we found that the introduction of aldehyde and methoxyl groups provide marked improvement for the inhibition against MRSA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang Q, Li HQ, Zong SC, Gao JM, Zhang AL. Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Rev Med Chem. 2012;12:127–48.
Li GY, Li BG, Yang T, Liu GY, Zhang GL. Secondary metabolites from the fungus Chaetomium brasiliense. Helv Chim Acta. 2008;91:124–9.
Khumkomkhet P, Kanokmedhakul S, Kanokmedhakul K, Hahnvajanawong C, Soytong K. Antimalarial and cytotoxic depsidones from the fungus Chaetomium brasiliense. J Nat Prod. 2009;72:1487–91.
Oh H, Swenson DC, Gloer JB, Wicklow DT, Dowd PF. Chaetochalasin A: a new bioactive metabolite from Chaetomium brasiliense. Tetrahedron Lett. 1998;39:7633–6.
Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1:18–18.
Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–43.
Wang QX, Bao L, Yang XL, Guo H, Yang RN, Ren B, et al. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia. 2012;83:209–14.
Ouyang J, Mao Z, Guo H, Xie Y, Cui Z, Sun J, et al. Mollicellins O-R, four new depsidones isolated from the endophytic fungus Chaetomium sp. Eef-10. Molecules. 2018;23:3218.
Cai R, Chen S, Long Y, Li C, Huang X, She Z. Depsidones from Talaromyces stipitatus SK-4, an endophytic fungus of the mangrove plant Acanthus ilicifolius. Phytochem Lett. 2017;20:196–9.
Niu S, Liu D, Hu X, Proksch P, Shao Z, Lin W. Spiromastixones A–O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J Nat Prod. 2014;77:1021–30.
Saetang P, Rukachaisirikul V, Phongpaichit S, Preedanon S, Sakayaroj J, Borwornpinyo S, et al. Depsidones and an α-pyrone derivative from Simpilcillium sp. PSU-H41, an endophytic fungus from Hevea brasiliensis leaf. Phytochemistry. 2017;143:115–23.
Sisodia R, Geol M, Verma S, Rani A, Dureja P. Antibacterial and antioxidant activity of lichen species Ramalina roesleri. Nat Prod Res. 2013;27:2235–9.
Sultana N, Afolayan AJ. A new depsidone and antibacterial activities of compounds from Usnea undulata Stirton. J Asian Nat Prod Res. 2011;13:1158–64.
Sweidan A, Chollet-Krugler M, Sauvager A, van de Weghe P, Chokr A, Bonnaure-Mallet M, et al. Antibacterial activities of natural lichen compounds against Streptococcus gordonii and Porphyromonas gingivalis. Fitoterapia. 2017;121:164–9.
Armaleo D, Sun X, Culberson C. Insights from the first putative biosynthetic gene cluster for a lichen depside and depsidone. Mycologia. 2011;103:741–54.
Kroken S, Glass NL, Taylor JW, Yoder OC, Turgeon BG. Phylogenomic analysis of type I polyketide synthase genes in pathogenic and saprobic ascomycetes. Proc Natl Acad Sci USA. 2003;100:15670–5.
Ibrahim SRM, Mohamed GA, Al Haidari RA, El-Kholy AA, Zayed MF, Khayat MT. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia. 2018;129:317–65.
Klaiklay S, Rukachaisirikul V, Aungphao W, Phongpaichit S, Sakayaroj J. Depsidone and phthalide derivatives from the soil-derived fungus Aspergillus unguis PSU-RSPG199. Tetrahedron Lett. 2016;57:4348–51.
Zhang Y, Mu J, Feng Y, Wen L, Han J. Four chlorinated depsidones from a seaweed-derived strain of Aspergillus unguis and their new biological activities. Nat Prod Res. 2014;28:503–6.
Acknowledgements
We gratefully acknowledge the financial support of the National Key R&D Program of China (Nos. 2017YFF0209900, 2017YFF0209904, 2017YFF0209902), National Natural Science Foundation of China (31770024), Qilu University of Technology (Shandong Academy of Sciences) International Cooperation Program (2019GHPY07), the Taishan Scholar Project from Shandong Province to Lixin Zhang, Xuekui Xia, Qilu University of Technology (Shandong Academy of Sciences) Joint Fund for Young Doctors (2017BSH2016), Science, Education and Industry Integration Innovation Pilot Project of Qilu University of Technology (Shandong Academy of Sciences) (2020KJC-ZD08).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, P., Yang, M., Zhu, G. et al. Mollicellins S-U, three new depsidones from Chaetomium brasiliense SD-596 with anti-MRSA activities. J Antibiot 74, 317–323 (2021). https://doi.org/10.1038/s41429-020-00398-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-00398-8
This article is cited by
-
Identification and characterization of a novel decalin derivative with anti-Candida activity from Streptomyces chrestomyceticus strain ADP4
Archives of Microbiology (2024)
-
Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): an updated review from 2016 to 2021
Mycological Progress (2021)