Abstract
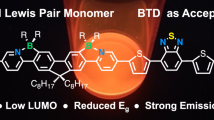
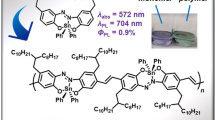
We previously reported that functionalized phenyl- and vinyl-silsesquioxanes (SQs) and [RSiO1.5]8,10,12 (R = Ph or vinyl) exhibited redshifted absorption and emission, suggesting 3-D conjugation via a cage-centered lowest unoccupied molecular orbital (LUMO). The functionalized [PhSiO1.5]7(OSiMe3)3 with a missing corner and edge-opened, end-capped [PhSiO1.5]8(OSiMe2)2 (double decker, DD) analogs also exhibit emission redshifts, indicating 3-D conjugation. DD [PhSiO1.5]8(OSiMevinyl)2 and R-Ar-Br copolymers exhibit polymerization (DP)-dependent emission λmax and integer charge transfer (ICT) to 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane (F4TNCQ). The terpolymer-averaged redshifts all suggest conjugation with two (O-Si-O) endcaps, possibly via a cage-centered LUMO. In assessing conjugation limits, it was anticipated that copolymers of the ladder (LL) SQ, (vinylMeSiO2)[PhSiO1.5]4(O2SiMevinyl), with Br-Ar-Br and without a cage would eliminate LUMO formation and a redshift. The λmax values observed were greater for analogous copolymers, which requires a different explanation. Here, we assess the photophysical behavior of copolymers closer to polysiloxanes, namely, the expanded cage (MeVinylSiO)2[PhSiO1.5]8(OSiMeVinyl)2SQs. Copolymers with Br-Ar-Br exhibit redshifted absorption and emission, which supports conjugation via Si-O-Si bonds rather than cage-centered LUMOs, contrary to traditional views of Si-O-Si copolymers. One- and two-photon photophysical probes showed that XDD copolymers exhibit multiple fluorescence-emitting excited states, in violation of Kasha’s rule stating that emission should occur only from the lowest excited state. Finally, new modeling studies suggested that conjugation derives from Si-O-Si bond dπ-pπ interactions, an unexpected result for polysiloxanes that supports two forms of conjugation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
West R. Multiple bonds to silicon: 20 years later. Polyhedron. 2002;21:467–72. https://doi.org/10.1016/S0277-5387(01)01017-8
Raabe G, Michl J. Multiple bonding to silicon. Chem Rev. 1985;85:419–509. https://doi.org/10.1021/cr00069a005
Baceiredo A, Kato T. Multiple bonds to silicon (recent advances in the chemistry of silicon containing multiple bonds). In Organosilicon Compounds; Elsevier, 2017; pp 533–618. https://doi.org/10.1016/B978-0-12-801981-8.00009-5
Boudin A, Cerveau G, Chuit C, Corriu RJP, Reye C. Reactivity of dianionic hexacoordinated silicon complexes toward nucleophiles: a new route to organosilanes from silica. Organometallics. 1988;7:1165–71. https://doi.org/10.1021/om00095a023
Laine RM, Blohowiak KY, Robinson TR, Hoppe ML, Nardi P, Kampf J, Uhm J. Synthesis of pentacoordinate silicon complexes from SiO2. Nature. 1991;353:642–4. https://doi.org/10.1038/353642a0
Chuit C, Corriu RJP, Reye C, Young JC. Reactivity of penta- and hexacoordinate silicon compounds and their role as reaction intermediates. Chem Rev. 1993;93:1371–448. https://doi.org/10.1021/cr00020a003
Kost D, Kalikhman I. Hypercoordinate silicon complexes based on hydrazide ligands. A remarkably flexible molecular system. Acc Chem Res. 2009;42:303–14. https://doi.org/10.1021/ar800151k
Kocher N, Henn J, Gostevskii B, Kost D, Kalikhman I, Engels B, Stalke D. Si−E (E = N, O, F) bonding in a hexacoordinated silicon complex: new facts from experimental and theoretical charge density studies. J Am Chem Soc. 2004;126:5563–8. https://doi.org/10.1021/ja038459r
Fujimoto H, Yabuki T, Tamao K, Fukui K. A theoretical study of chemical bonds in silicon species. J Mol Struct. 1992;260:47–61. https://doi.org/10.1016/0166-1280(92)87034-W
Yamaguchi S, Tamao K. Silole-containing σ- and π-conjugated compounds. J Chem Soc Dalton Trans. 1998, 3693–702. https://doi.org/10.1039/a804491k
Kumar VB, Leitao EM. Properties and applications of polysilanes. Appl Organo Chem. 2020;34:e5402 https://doi.org/10.1002/aoc.5402
Qin Y, Chen H, Yao J, Zhou Y, Cho Y, Zhu Y, Qiu B, Ju C-W, Zhang Z-G, He F, Yang C, Li Y, Zhao D. Silicon and oxygen synergistic effects for the discovery of new high-performance nonfullerene acceptors. Nat Commun. 2020;11:5814 https://doi.org/10.1038/s41467-020-19605-z
Chen J, Cao Y. Silole‐containing polymers: chemistry and optoelectronic properties. Macromol Rapid Commun. 2007;28:1714–42. https://doi.org/10.1002/marc.200700326
Voronkov MG, Lavrent’yev VI. Polyhedral Oligosilsesquioxanes and Their Homo Derivatives. In Inorganic Ring Systems; Boschke FL, Dewar MJS, Dunitz JD, Hafner K, Heilbronner E, Itô S, Lehn J-M, Niedenzu K, Raymond KN, Rees CW, Schäfer K, Vögtle F, Wittig G, Series Eds.; Topics in Current Chemistry; Springer Berlin Heidelberg: Berlin, Heidelberg, 1982; Vol. 102, pp 199–236. https://doi.org/10.1007/3-540-11345-2_12
Schwab JJ, Lichtenhan JD, Chaffee KP, Mather PT, Romo-Uribe A. Polyhedral oligomeric silsesquioxanes (poss): silicon based monomers and their use in the preparation of hybrid polyurethanes. MRS Proc. 1998;519:21 https://doi.org/10.1557/PROC-519-21
Baney RH, Itoh M, Sakakibara A, Suzuki T. Silsesquioxanes. Chem Rev 1995;95:1409–30. https://doi.org/10.1021/cr00037a012
Calzaferri GS. In Tailor-made Silicon-Oxygen Compounds; Friedr. Vieweg & SohnmbH, 1996; pp 149-69.
Lichtenhan J. Silsesquioxane-based polymers. In Polymeric Materials Encyc.; CRC Press, N.Y, 1996; Vol. 10, pp 7768–77.
Provatas A, Matisons JG. Synthesis and applications of silsesquioxanes. In. Trends Polym Sci 1997;5:327–322.
Li G, Wang L, Ni H, Pittman CU,Jr. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: a review. J Inorg Organomet Polym. 2001;11:123–54. https://doi.org/10.1023/A:1015287910502
Duchateau R. Incompletely condensed silsesquioxanes: versatile tools in developing silica-supported olefin polymerization catalysts. Chem Rev 2002;102:3525–42. https://doi.org/10.1021/cr010386b
Abe Y, Gunji T. Oligo- and polysiloxanes. Prog Polym Sci. 2004;29:149–82. https://doi.org/10.1016/j.progpolymsci.2003.08.003
Phillips SH, Haddad TS, Tomczak SJ. Developments in nanoscience: polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr Opin Solid State Mater Sci. 2004;8:21–29. https://doi.org/10.1016/j.cossms.2004.03.002
Kannan RY, Salacinski HJ, Butler PE, Seifalian AM. Polyhedral oligomeric silsesquioxane nanocomposites: the next generation material for biomedical applications. Acc Chem Res. 2005;38:879–84. https://doi.org/10.1021/ar050055b
Laine RM. Nanobuilding blocks based on the [OSiO1.5]x (x = 6, 8, 10) octasilsesquioxanes. J Mater Chem. 2005;15:3725. https://doi.org/10.1039/b506815k
Lickiss PD, Rataboul F. Fully condensed polyhedral oligosilsesquioxanes (POSS): from synthesis to application. Adv Organomet Chem. 2008;57:1–116. https://doi.org/10.1016/S0065-3055(08)00001-4
Chan KL, Sonar P, Sellinger A. Cubic silsesquioxanes for use in solution processable organic light emitting diodes (OLED). J Mater Chem. 2009;19:9103 https://doi.org/10.1039/b909234j
Wu J, Mather PT. POSS polymers: physical properties and biomaterials applications. Polym Rev. 2009;49:25–63. https://doi.org/10.1080/15583720802656237
Cordes DB, Lickiss PD, Rataboul F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev. 2010;110:2081–173. https://doi.org/10.1021/cr900201r
Laine RM, Roll MF. Polyhedral Phenylsilsesquioxanes. Macromolecules. 2011;44:1073–109. https://doi.org/10.1021/ma102360t
Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C., Ed.; Advances in silicon science; Springer: Dordrecht, 2011.
McCabe C, Glotzer SC, Kieffer J, Neurock M, Cummings PT. Multiscale simulation of the synthesis, assembly and properties of nanostructured organic/inorganic hybrid materials. J Comput Theor Nanosci. 2004;1:265–79. https://doi.org/10.1166/jctn.2004.024
Ionescu TC, Qi F, McCabe C, Striolo A, Kieffer J, Cummings PT. Evaluation of force fields for molecular simulation of polyhedral oligomeric silsesquioxanes. J Phys Chem B. 2006;110:2502–10. https://doi.org/10.1021/jp052707j
Bassindale AR, Pourny M, Taylor PG, Hursthouse MB, Light ME. Fluoride-ion encapsulation within a silsesquioxane cage. Angew Chem Int Ed 2003;42:3488–90. https://doi.org/10.1002/anie.200351249
Anderson SE, Bodzin DJ, Haddad TS, Boatz JA, Mabry JM, Mitchell C, Bowers MT. Structural investigation of encapsulated fluoride in polyhedral oligomeric silsesquioxane cages using ion mobility mass spectrometry and molecular mechanics. Chem Mater 2008;20:4299–309. https://doi.org/10.1021/cm800058z
Guan J, Tomobe K, Madu I, Goodson T III, Makhal K, Trinh MT, et al. Photophysical properties of partially functionalized phenylsilsesquioxane: [RSiO1.5]7[Me/nPrSiO1.5] and [RSiO1.5]7[O0.5SiMe3]3 (R = 4-Me/4-CN-Stilbene). Cage-centered magnetic fields form under intense laser light. Macromolecules. 2019;52:4008–19. https://doi.org/10.1021/acs.macromol.9b00699
Laine RM, Sulaiman S, Brick C, Roll M, Tamaki R, Asuncion MZ, Neurock M, Filhol J-S, Lee C-Y, Zhang J, Goodson T, Ronchi M, Pizzotti M, Rand SC, Li Y. Synthesis and photophysical properties of stilbeneoctasilsesquioxanes. emission behavior coupled with theoretical modeling studies suggest a 3-d excited state involving the silica core. J Am Chem Soc 2010;132:3708–22. https://doi.org/10.1021/ja9087709
Guan J, Tomobe K, Madu I, Goodson T, Makhal K, Trinh MT, et al. Photophysical Properties of Functionalized Double Decker Phenylsilsesquioxane Macromonomers: [PhSiO1.5]8[OSiMe2)2 and [PhSiO1.5]8(O0.5SiMe3)4. Cage-Centered Lowest Unoccupied Molecular Orbitals Form Even When Two Cage Edge Bridges Are Removed, Verified by Modeling and Ultrafast Magnetic Light Scattering Experiments. Macromolecules. 2019;52:7413–22. https://doi.org/10.1021/acs.macromol.9b00700
Guan J, Arias JJR, Tomobe K, Ansari R, Marques MdeFV, Rebane A, et al. Unconventional Conjugation via vinylMeSi(O−)2 Siloxane Bridges May Imbue Semiconducting Properties in [Vinyl(Me)SiO(PhSiO1.5)8OSi(Me)Vinyl-Ar] Double-Decker Copolymers. ACS Appl Polym Mater 2020;2:3894–907. https://doi.org/10.1021/acsapm.0c00591
Guan J, Arias JJR, Tomobe K, Ansari R, Marques M de FV, Rebane A, et al. Unconventional Conjugation via vinylMeSi(O−)2 Siloxane Bridges May Imbue Semiconducting Properties in [Vinyl(Me)SiO(PhSiO1.5)8 OSi(Me)Vinyl-Ar] Double-Decker Copolymers. ACS Appl Polym Mater 2020, acsapm.0c00591. https://doi.org/10.1021/acsapm.0c00591
Guan J, Sun Z, Ansari R, Liu Y, Endo A, Unno M, Ouali A, Mahbub S, Furgal JC, Yodsin N, Jungsuttiwong S, Hashemi D, Kieffer J, Laine RM. Conjugated copolymers that shouldn’t be. Angew Chem Int Ed. 2021;60:11115–9. https://doi.org/10.1002/anie.202014932
Asuncion MZ, Laine RM. Fluoride rearrangement reactions of polyphenyl- and polyvinylsilsesquioxanes as a facile route to mixed functional phenyl, Vinyl T 10 and T 12 silsesquioxanes. J Am Chem Soc. 2010;132:3723–36. https://doi.org/10.1021/ja9087743
Jung JH, Furgal JC, Clark S, Schwartz M, Chou K, Laine RM. Beads on a Chain (BoC) Polymers with Model Dendronized Beads. Copolymerization of [(4-NH2PhSiO1.5)6(IPhSiO1.5)2] and [(4-CH3OPhSiO1.5)6(IPhSiO1.5)2] with 1,4-Diethynylbenzene (DEB) Gives Through-Chain, Extended 3-D Conjugation in the Excited State That Is an Average of the Corresponding Homopolymers. Macromolecules. 2013;46:7580–90. https://doi.org/10.1021/ma401422t
Zhang Z, Guan J, Ansari R, Kieffer J, Yodsin N, Jungsuttiwong S, et al. Further proof of unconventional conjugation via disiloxane bonds: double decker sesquioxane [vinylMeSi(O0.5)2(PhSiO1.5)8(O0.5)2SiMevinyl] derived alternating terpolymers give excited-state conjugation averaging that of the corresponding copolymers. Macromolecules. 2022, acs.macromol.2c01355. https://doi.org/10.1021/acs.macromol.2c01355
Liu Y, Takeda N, Ouali A, Unno M. Synthesis, characterization, and functionalization of tetrafunctional double-decker siloxanes. Inorg Chem 2019;58:4093–8. https://doi.org/10.1021/acs.inorgchem.9b00416
Endo H, Takeda N, Unno M. Synthesis and properties of phenylsilsesquioxanes with ladder and double-decker structures. Organometallics. 2014;33:4148–51. https://doi.org/10.1021/om500010y
Demchenko AP, Tomin VI, Chou P-T. Breaking the Kasha rule for more efficient photochemistry. Chem Rev. 2017;117:13353–81. https://doi.org/10.1021/acs.chemrev.7b00110
del Valle JC, Catalán J. Kasha’s rule: a reappraisal. Phys Chem Chem Phys. 2019;21:10061–9. https://doi.org/10.1039/C9CP00739C
Guan J, Tomobe K, Madu I, Goodson T, Makhal K, Trinh MT, et al. Photophysical Properties of Partially Functionalized Phenylsilsesquioxane: [RSiO1.5]7[Me/nPrSiO1.5] and [RSiO1.5]7[O0.5SiMe3]3 (R = 4-Me/4-CN-Stilbene). Cage-Centered Magnetic Fields Form under Intense Laser Light. Macromolecules. 2019;52:4008–19. https://doi.org/10.1021/acs.macromol.9b00699
Dankert F, Hänisch C. Siloxane coordination revisited: Si−O bond character, reactivity and magnificent molecular shapes. Eur J Inorg Chem. 2021;2021:2907–27. https://doi.org/10.1002/ejic.202100275
Fugel M, Hesse MF, Pal R, Beckmann J, Jayatilaka D, Turner MJ, Karton A, Bultinck P, Chandler GS, Grabowsky S. Covalency and ionicity do not oppose each other—relationship between Si−O bond character and basicity of siloxanes. Chem Eur J. 2018;24:15275–86. https://doi.org/10.1002/chem.201802197
Sulaiman S, Bhaskar A, Zhang J, Guda R, Goodson T, Laine RM. Molecules with perfect cubic symmetry as nanobuilding blocks for 3-D assemblies. elaboration of octavinylsilsesquioxane. Unusual luminescence shifts may indicate extended conjugation involving the silsesquioxane core. Chem Mater. 2008;20:5563–73. https://doi.org/10.1021/cm801017e
Sulaiman S, Zhang J, Goodson T III, Laine RM. Synthesis, characterization and photophysical properties of polyfunctional phenylsilsesquioxanes: [O-RPhSiO1.5]8, [2,5-R2PhSiO1.5]8, and [R3PhSiO1.5]8. Compounds with the highest number of functional units/unit volume. J Mater Chem. 2011;21:11177. https://doi.org/10.1039/c1jm11701g
Furgal JC, Jung JH, Clark S, Goodson T, Laine RM. Beads on a chain (BoC) phenylsilsesquioxane (SQ) polymers via F − catalyzed rearrangements and ADMET or reverse heck cross-coupling reactions: through chain, extended conjugation in 3-D with potential for dendronization. Macromolecules. 2013;46:7591–604. https://doi.org/10.1021/ma401423f
Bahrami M, Hashemi H, Ma X, Kieffer J, Laine RM. Why Do the [PhSiO 1.58,10,12> Cages self-brominate primarily in the ortho position? modeling reveals a strong cage influence on the mechanism. Phys Chem Chem Phys. 2014;16:25760–4. https://doi.org/10.1039/C4CP03997A
Zhang Z, Kaehr H, Laine RM. Polysiloxane copolymers demonstrate conjugation through Si-O-Si bonds, 2023.
Z Zhang; JJR Arias; H Kaehr,; Y Liu; M Takahashi; R Murata, et al. Conjugation through Si-O-Si bonds, extended examples via SiO0.5/SiO1.5 units. Multiple emissive states in violation of Kasha’s rule., TBD.
Acknowledgements
The Laine and Rebane groups gratefully thank NSF Chemistry for the collaborative research award No. 1610344. Support from the Estonian National Science Foundation grant PRG661 is acknowledged (Ramo and Rebane). The Unno/Liu group is grateful for support from the NEDO project (JPNP06046). Professor Jungsuttiwong thanks NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [B16F640099] for funding work performed by her team. The work performed at The Georgia Institute of Technology was made possible through the Air Force Office of Scientific Research (AFOSR) under support provided by the Organic Materials Chemistry Program (Grant FA9550-20-1-0353, Program Manager: Dr. Kenneth Caster).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arias, J.J.R., Zhang, Z., Takahashi, M. et al. Conjugation in polysiloxane copolymers via unexpected Si-O-Si dπ-pπ overlap, a second mechanism?. Polym J (2024). https://doi.org/10.1038/s41428-024-00899-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-024-00899-5