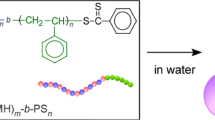
Abstract
A polymer with a hydrophilic group, such as poly(methyl methacrylate) (PMMA), spreads as a monolayer on a water surface, whereas a polymer without a hydrophilic group, such as polystyrene (PS), does not spread but instead forms aggregates that are much thicker than the monolayer. Previously, we spread PS-b-PMMA from a very dilute solution to avoid intermolecular aggregation and found that a single PS block aggregated to form a PS single-block particle from which a PMMA monolayer emanated. In this study, we investigated whether PS-b-PMMA-b-PS could be used to prepare PS single-block particles at both ends without aggregation of the PS blocks. We used mixed monolayers with a small amount of PS-b-PMMA-b-PS and a large amount of poly(n-nonyl acrylate) (PNA) or PMMA. Both PNA and PMMA form miscible monolayers with the middle PMMA block, preventing the PS blocks from aggregating at both ends. PS-b-PMMA-b-PS was successfully solubilized as isolated chains in the matrix monolayers with PS single-block particles at both ends. We evaluated the end-to-end distance using the PS single-block particles at the ends and found that the PMMA block chain was not highly segregated in the PMMA monolayer but rather was elongated and interpenetrated with the matrix chains.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gaines GL Insoluble monolayers at liquid-gas interfaces. New York: Interscience; 1966.
Ulman A An introduction to ultrathin organic films: from Langmuir–Blodgett to self-assembly. New York: Academic Press; 1991.
Zhu J, Eisenberg A, Lennox RB. Interfacial behavior of block polyelectrolytes 1. Evidence for novel surface micelle formation. J Am Chem Soc. 1991;113:5583–88.
Zhu J, Lennox RB, Eisenberg AJ. Polymorphism of (quasi) 2-dimensional micelles. J Phys Chem. 1992;96:4727–30.
Zhu J, Eisenberg A, Lennox RB. Interfacial behavior of block polyelectrolytes 5. Effect of varying block lengths on the properties of surface micelles. Macromolecules. 1992;25:6547–55.
Li S, Hanley S, Khan I, Varshney SK, Eisenberg A, Lennox RB. Surface micelle formation at the air/water interface from nonionic diblock copolymers. Langmuir. 1993;9:2243–6.
Cheyne RB, Moffitt MG. Novel two-dimensional “ring and chain” morphologies in Langmuir−Blodgett monolayers of PS-b-PEO block copolymers: effect of spreading solution concentration on self-assembly at the air−water interface. Langmuir. 2005;21:5253–60.
Lu Q, Bazuin CG. Solvent-assisted formation of nanostrand networks from supramolecular diblock copolymer/surfactant complexes at the air/water interface. Nano Lett. 2005;5:1309–14.
Kumaki J, Nishikawa Y, Hashimoto T. Visualization of single chain conformations of a synthetic polymer with atomic force microscopy. J Am Chem Soc. 1996;118:3321–22.
Kumaki J, Hashimoto T. Conformational change in isolated single synthetic polymer chain on mica surface observed by atomic force microscopy. J Am Chem Soc. 2003;125:4907–17.
Sugihara K, Kumaki J. Visualization of two-dimensional single chain conformations solubilized in miscible polymer blend monolayer by atomic force microscopy. J Phys Chem B. 2012;116:6561–68.
Brandrup J, Immergut EH, Grulke EA, editors. Polymer handbook, 4th ed. New York: Wiley-Interscience Publication; 1999.
de Gennes P-G Scaling concepts in polymer physics. London: Cornell University Press; 1979.
Mayer H, Kreer T, Aichele M, Cavallo A, Johner A, Baschnagel J, et al. Perimeter length and form factor in two-dimensional polymer melts. Phys Rev E. 2009;79:050802–1-4.
Mayer H, Wittmer JP, Kreer T, Johner A, Baschnagel J. Static properties of polymer melts in two dimensions. J Chem Phys. 2010;132:184904–1-12.
Aoki H, Morita S, Sekine R, Ito S. Conformation of single poly(methyl methacrylate) chains in an ultra-thin film studied by scanning near-field optical microscopy. Polym J. 2008;40:274–80.
Aoki H, Anryu M, Ito S. Two-dimensional polymers investigated by scanning near-field optical microscopy: conformation of single polymer chain in monolayer. Polymer. 2005;46:5896–902.
Aoki H, Mori K, Takahashi T, Ito S. Quantitative analysis of end-to-end distance of single polymer chain in ultra-thin film by super-resolution fluorescence imaging. Chem Phys. 2012;419:54–8.
Yethiraj A. Computer simulation study of two-dimensional polymer solutions. Macromolecules. 2003;36:5854–62.
Kim JM. Influence of chain stiffness on semiflexible polymer melts in two dimensions via molecular dynamics simulation. Mol Simul. 2021;47:1290–8.
Wang X, Folts VJ. Chain conformation in two-dimensional dense state. J Chem Phys. 2004;121:8158–62.
Kumaki J, Kajitani T, Nagai K, Okoshi K, Yashima E. Visualization of polymer chain conformations in amorphous polyisocyanide Langmuir–Blodgett films by atomic force Microscopy. J Am Chem Soc. 2010;132:5604–6.
Gallyamov MO, Tartsh B, Potemkin II, Börner HG, Matyjaszewski K, Khokhlov AR, et al. Individual bottle brush molecules in dense 2D layers restoring high degree of extension after collapse-decollapse cycle: directly measured scaling exponent. Eur Phys J E. 2009;29:73–85.
Kurata M, Stockyamer WH. Intrinsic viscosities and unperturbed dimensions of long chain molecules. Fortschr Hochpolym Forsch. 1963;3:196–312.
Maestro A, Guzmán E, Chuliá R, Ortega F, Rubio RG, Miller R. Fluid to soft-glass transition in a quasi-2D system: thermodynamic and rheological evidences for a Langmuir monolayer. Phys Chem Chem Phys. 2011;13:9534–9.
Arriaga LR, Monroy F, Langevin D. The polymer glass transition in nanometric films. EPL (Europhys Lett). 2012;98:38007–1-6.
Schulmann N, Mayer H, Kreer T, Cavallo A, Johner A, Baschnagel J, et al. Strictly two-dimensional self-avoiding walks: density crossover scaling. Polym Sci Ser C. 2013;55:181–211.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP25107706, JP20H05201, JP21H01993, and JP21K18993.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Honma, F., Kumaki, J. Solubilization of poly(styrene)(PS)-b-poly(methyl methacrylate)(PMMA)-b-PS in poly(n-nonyl acrylate) and PMMA monolayers as isolated chains with both PS blocks forming separated single-block particles. Polym J 54, 687–696 (2022). https://doi.org/10.1038/s41428-022-00614-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00614-2