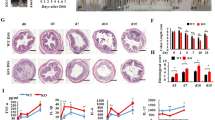
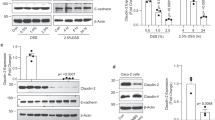
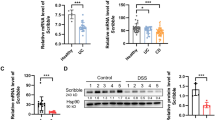
Abstract
IκB kinase (IKK) complex is central regulators of the NF-κB pathway, and dysregulation of IKK phosphorylation leads to hyperactivation of proinflammatory response in various chronic inflammatory diseases, including inflammatory bowel disease (IBD). However, the dynamic modulation of IKK phosphorylation and dephosphorylation in intestinal inflammation remains uncharacterized. Here, we found that autophagy/beclin-1 regulator 1 (AMBRA1) was highly expressed in inflamed colons in a colitis mouse model and in clinical IBD samples. Importantly, AMBRA1 deletion significantly decreased proinflammatory cytokine expression and enhanced the therapeutic effect of infliximab on intestinal inflammation. Mechanistically, the N-term F1 domain of AMBRA1 was required for AMBRA1 to competitively interact with protein phosphatase 4 regulatory subunit 1 (PP4R1) and catalytic protein phosphatase 4 (PP4c) to suppress their interactions with IKK, promote the dissociation of the PP4R1/PP4c complex, and antagonize the dephosphorylation activity of this complex towards the IKK complex. In response to TNF-α stimulation, IKKα phosphorylates AMBRA1 at S1043 to stabilize AMBRA1 expression by impairing its binding to Cullin4A (CUL4A) to decrease its CUL4A-mediated K48-linked ubiquitination. Overall, our study identifies an autophagy-independent function of AMBRA1 as a positive modulator of IKK phosphorylation to promote intestinal inflammation, thus providing a new targeted therapeutic strategy for patients with refractory IBD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152:313–321.e312.
Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053–60.
Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306.
Wong U, Cross RK. Primary and secondary nonresponse to infliximab: mechanisms and countermeasures. Expert Opin Drug Metabol Toxicol. 2017;13:1039–46.
Marchioni Beery R, Kane S. Current approaches to the management of new-onset ulcerative colitis. Clin Exp Gastroenterol. 2014;7:111–32.
Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–84.
Liu P, Li Y, Wang W, Bai Y, Jia H, Yuan Z, et al. Role and mechanisms of the NF-ĸB signaling pathway in various developmental processes. Biomed Pharmacother = Biomedecine & pharmacotherapie. 2022;153:113513.
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Pers Biol. 2009;1:a001651.
Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Sig Transduc Targeted Ther. 2020;5:209.
Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, et al. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat Cell Biol. 2009;11:659–66.
Sun SC, Maggirwar SB, Harhaj E. Activation of NF-kappa B by phosphatase inhibitors involves the phosphorylation of I kappa B alpha at phosphatase 2A-sensitive sites. The J Biol Chem. 1995;270:18347–51.
Li S, Wang L, Berman MA, Zhang Y, Dorf ME. RNAi screen in mouse astrocytes identifies phosphatases that regulate NF-kappaB signaling. Mol cell. 2006;24:497–509.
Weng T, Koh CG. POPX2 phosphatase regulates apoptosis through the TAK1-IKK-NF-κB pathway. Cell death Dis. 2017;8:e3051.
Brautigan DL, Shenolikar S. Protein Serine/Threonine Phosphatases: Keys to Unlocking Regulators and Substrates. Ann Rev Biochem. 2018;87:921–64.
Cohen PT, Philp A, Vázquez-Martin C. Protein phosphatase 4–from obscurity to vital functions. FEBS Lett. 2005;579:3278–86.
Brechmann M, Mock T, Nickles D, Kiessling M, Weit N, Breuer R, et al. A PP4 holoenzyme balances physiological and oncogenic nuclear factor-kappa B signaling in T lymphocytes. Immunity. 2012;37:697–708.
Cianfanelli V, De Zio D, Di Bartolomeo S, Nazio F, Strappazzon F, Cecconi F. Ambra1 at a glance. J Cell Sci. 2015;128:2003–8.
Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–16.
Dere E, Dahm L, Lu D, Hammerschmidt K, Ju A, Tantra M, et al. Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender. Front Behav Neurosci. 2014;8:181.
Heinrich A, Nees F, Lourdusamy A, Tzschoppe J, Meier S, Vollstädt-Klein S, et al. From gene to brain to behavior: schizophrenia-associated variation in AMBRA1 alters impulsivity-related traits. Eur J Neurosci. 2013;38:2941–5.
Liu J, Yuan B, Cao J, Luo H, Gu S, Zhang M, et al. AMBRA1 Promotes TGFβ Signaling via Nonproteolytic Polyubiquitylation of Smad4. Cancer Res. 2021;81:5007–20.
Di Leo L, Bodemeyer V, Bosisio FM, Claps G, Carretta M, Rizza S, et al. Loss of Ambra1 promotes melanoma growth and invasion. Nat Commun. 2021;12:2550.
Foerster EG, Mukherjee T, Cabral-Fernandes L, Rocha JDB, Girardin SE, Philpott DJ. How autophagy controls the intestinal epithelial barrier. Autophagy. 2022;18:86–103.
Rufini S, Ciccacci C, Di Fusco D, Ruffa A, Pallone F, Novelli G, et al. Autophagy and inflammatory bowel disease: Association between variants of the autophagy-related IRGM gene and susceptibility to Crohn’s disease. Digestive liver Dis.: Off J Italian Soc Gastroenterol Italian Assoc Stud Liver. 2015;47:744–50.
Holik AZ, Young M, Krzystyniak J, Williams GT, Metzger D, Shorning BY, et al. Brg1 loss attenuates aberrant wnt-signalling and prevents wnt-dependent tumourigenesis in the murine small intestine. PLoS Genet. 2014;10:e1004453.
Takada Y, Fukuda A, Chiba T, Seno H. Brg1 plays an essential role in development and homeostasis of the duodenum through regulation of Notch signaling. Development (Cambridge, England). 2016;143:3532–9.
Liu M, Sun T, Li N, Peng J, Fu D, Li W, et al. BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration. Nat Commun. 2019;10:4614.
Cianfanelli V, Fuoco C, Lorente M, Salazar M, Quondamatteo F, Gherardini PF, et al. AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-Myc dephosphorylation and degradation. Nat Cell Biol. 2015;17:20–30.
Maiani E, Milletti G, Nazio F, Holdgaard SG, Bartkova J, Rizza S, et al. AMBRA1 regulates cyclin D to guard S-phase entry and genomic integrity. Nature. 2021;592:799–803.
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75.
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021;17:1–382.
Di Rita A, Peschiaroli A, D Acunzo P, Strobbe D, Hu Z, Gruber J, et al. HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKα. Nat Commun. 2018;9:3755.
Huttlin EL, Bruckner RJ, Navarrete-Perea J, Cannon JR, Baltier K, Gebreab F, et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell. 2021;184:3022–3040.e3028.
Antonioli M, Albiero F, Nazio F, Vescovo T, Perdomo AB, Corazzari M, et al. AMBRA1 interplay with cullin E3 ubiquitin ligases regulates autophagy dynamics. Develop Cell. 2014;31:734–46.
Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323–37.
Nazio F, Po A, Abballe L, Ballabio C, Diomedi Camassei F, Bordi M, et al. Targeting cancer stem cells in medulloblastoma by inhibiting AMBRA1 dual function in autophagy and STAT3 signalling. Acta neuropathologica. 2021;142:537–64.
Netea-Maier RT, Plantinga TS, van de Veerdonk FL, Smit JW, Netea MG. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy. 2016;12:245–60.
Schwerd T, Pandey S, Yang HT, Bagola K, Jameson E, Jung J, et al. Impaired antibacterial autophagy links granulomatous intestinal inflammation in Niemann-Pick disease type C1 and XIAP deficiency with NOD2 variants in Crohn’s disease. Gut. 2017;66:1060–73.
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang C, et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–60.
Wu H, Lu XX, Wang JR, Yang TY, Li XM, He XS, et al. TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/β-catenin degradation and is targeted for GSK3B/GSK3β-mediated phosphorylation and degradation. Autophagy. 2019;15:1506–22.
Di Rienzo M, Antonioli M, Fusco C, Liu Y, Mari M, Orhon I, et al. Autophagy induction in atrophic muscle cells requires ULK1 activation by TRIM32 through unanchored K63-linked polyubiquitin chains. Sci Adv. 2019;5:eaau8857.
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61.
Liang T, Song M, Xu K, Guo C, Xu H, Zhang H, et al. TRIM32 promotes inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. Scandinavian J Immunol. 2020;91:e12876.
Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol. 2014;20:64–77.
Ma, Iwamoto TY, Hoa GK, Akotia NT, Pedram V, Boivin MA A, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointestinal liver Physiol. 2004;286:G367–376.
Cornillie F, Hanauer SB, Diamond RH, Wang J, Tang KL, Xu Z, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–7.
Panaccione R, Colombel JF, Sandborn WJ, D’Haens G, Zhou Q, Pollack PF, et al. Adalimumab maintains remission of Crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Alimentary Pharmacol Therapeutics. 2013;38:1236–47.
Ben-Horin S, Chowers Y. Tailoring anti-TNF therapy in IBD: drug levels and disease activity. Nat Rev Gastroenterol Hepatol. 2014;11:243–55.
Steenholdt C, Brynskov J, Thomsen O, Munck LK, Fallingborg J, Christensen LA, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–27.
Xu W, Ding W, Gu Y, Cui L, Zhong J, Du P. Risk Factors of Colorectal Stricture Associated with Developing High-Grade Dysplasia or Cancer in Ulcerative Colitis: A Multicenter Long-term Follow-up Study. Gut liver. 2020;14:601–10.
Gu W, Wan D, Qian Q, Yi B, He Z, Gu Y, et al. Ambra1 is an essential regulator of autophagy and apoptosis in SW620 cells: pro-survival role of Ambra1. PloS one. 2014;9:e90151.
Maiani E, Milletti G, Cecconi F. The pro-autophagic protein AMBRA1 coordinates cell cycle progression by regulating CCND (cyclin D) stability. Autophagy. 2021;17:4506–8.
Simoneschi D, Rona G, Zhou N, Jeong YT, Jiang S, Milletti G, et al. CRL4(AMBRA1) is a master regulator of D-type cyclins. Nature. 2021;592:789–93.
Wei Z, Song J, Wang G, Cui X, Zheng J, Tang Y, et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun. 2018;9:4468.
Xu W, Guo Y, Huang Z, Zhao H, Zhou M, Huang Y, et al. Small heat shock protein CRYAB inhibits intestinal mucosal inflammatory responses and protects barrier integrity through suppressing IKKβ activity. Mucosal immunol. 2019;12:1291–303.
Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathologe. 1987;8:138–40.
Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–8.
Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterol. 2007;133:1099–105.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82270549, 82000481, 82372648, 82000494) and Natural Science Foundation of Shanghai (No. 22ZR1440500).
Author information
Authors and Affiliations
Contributions
Peng Du, Chen-Ying Liu and YingWei Chen designed the research. Weimin Xu, Zhebin Hua and Yaosheng Wang performed experiments and/or analyzed data. Weimin Xu and Chen-Ying Liu wrote the manuscript. Wenbo Tang, Weijun Ou, Fangyuan Liu, Yiqing Yang, Wenjun Ding and Wensong Ge assisted some analysis and collected the specimens from patients. Zhongchuan Wang, Long Cui, Yubei Gu and Xiaolei Wang reviewed and revised the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committee of Xinhua Hospital (No. XHEC-NSFC-2022-113). All mouse studies were approved, and all animals were manipulated according to the protocols approved by the Animal Care and Use Committees of Xinhua Hospital and animal care was conducted in accordance with institutional guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, W., Hua, Z., Wang, Y. et al. AMBRA1 promotes intestinal inflammation by antagonizing PP4R1/PP4c mediated IKK dephosphorylation in an autophagy-independent manner. Cell Death Differ (2024). https://doi.org/10.1038/s41418-024-01275-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-024-01275-9