Abstract
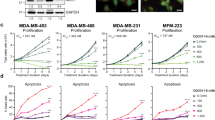
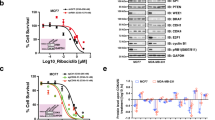
CDK4/6 inhibitors significantly prolong progression-free survival in patients with advanced hormone receptor-positive (HR+) HER2-negative breast cancer. Despite recent successes, patients acquire resistance, necessitating the development of additional novel therapeutic strategies. Bromodomain and extra-terminal domain (BET) proteins are key epigenetic regulators that interact with acetylated lysine (AcLys) residues of histones or transcription factors. BET proteins are directly involved in modulating estrogen receptor (ER) signaling and the cell cycle. Therefore, BET inhibitors can potentially offer new strategies in the treatment of advanced ER+ breast cancer. ZEN-3694 is an orally bioavailable small molecule BET inhibitor currently being evaluated in Phase 1/2 clinical trials (NCT03901469). To assess a potential combination strategy in a CDK4/6i resistant breast cancer population, we investigated the mechanism of action of ZEN-3694 combined with CDK4/6 inhibitors in the ER+ cell lines resistant to palbociclib or abemaciclib. Here, we describe that the combination of ZEN-3694 with CDK4/6i potently inhibits proliferation and induces apoptosis in CDK4/6i resistant cell lines. The resistance to both palbociclib and abemaciclib was associated with the strong upregulation of CDK6 and CCND1 protein levels, which was reversed by the ZEN-3694 treatment. Furthermore, RNAseq data and pathway analysis elucidated the combinatorial effects of ZEN-3694 with CDK4/6 inhibitors through significant downregulation of multiple pathways involved in cell cycle regulation, cellular growth, proliferation, apoptosis, inflammation, and cellular immune response. Our data indicate that ZEN-3694 has therapeutic potential in combination with CDK4/6 inhibitors in patients with advanced ER+ breast resistant to CDK4/6 inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr Relat Cancer. 2019;26:R-15–30.
Niu Y, Sun T. Cyclin-dependent kinases 4/6 inhibitors in breast cancer: current status, resistance, and combination strategies. J Cancer. 2019;10:5504–17.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35.
Guarducci C, Bonechi M, Benelli M, Biagioni C, Boccalini G, Romagnoli D, et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4:38.
Kettner NM, Vijayaraghavan S, Durak MG, Bui T, Kohansal M, Ha Min J, et al. Combined inhibition of STAT3 and DNA repair in palbociclib-resistant ER-positive breast cancer. Clin Cancer Res. 2019;25:3996–4013.
Kharenko OA, Patel RG, Jahagirdar R, Attwell S, Calosing C, Tsujikawa L, et al. The BET bromodomain inhibitors ZEN-3694 and ZEN-3309 targets several mechanisms of resistance to endocrine therapies in preclinical models of ER+ breast cancers. Cancer Res. 2018;78:P3–06-07.
Feng Q, Zhang Z, Shea MJ, Creighton CJ, Coarfa C, Hilsenbeck SG, et al. An epigenomic approach to therapy for tamoxifen-resistant breast cancer. Cell Res. 2014;24:809–19.
Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–64.
Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–13.
O’Brien N, Conklin D, Beckmann R, Luo T, Chau K, Thomas J, et al. Preclinical activity of abemaciclib alone or in combination with antimitotic and targeted therapies in breast cancer. Mol Cancer Ther. 2018;17:897–907.
Aiyengar TM, Chiranjeevi P, Rani HS. Role of endothelial nitric oxide synthase in breast cancer, Nitric Oxide Synthase: Simple Enzyme-Complex Roles, 2017, 179-196.
Du MJ, Chen XD, Zhou XL, Wan Y, Lan B, Zhang CZ, et al. Estrogen induces VAV1 expression in human breast cancer cells, PloS ONE, 2014, 9, e99052, 1-12.
Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34:893–905.
Liang LY, Patel O, Janes PW, Murphy JM, Lucet IS. Eph receptor signalling: from catalytic to non-catalytic functions. Oncogene. 2019;38:6567–84.
Pancholi S, Ribas R, Simigdala N, Schuster E, Nikitorowicz-Buniak J, Ressa A, et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene. 2020;39:4781–97.
Haga RB, Ridley AJ. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–21.
Harrington WR, Sengupta S, Katzenellenbogen BS. Estrogen regulation of the glucuronidation enzyme UGT2B15 in estrogen receptor-positive breast cancer cells. Endocrinology. 2006;147:1–3850.
Qu Y, Hao C, Xu J, Cheng Z, Wang W, Liu H. ILK promotes cell proliferation in breast cancer cells by activating the PI3K/Akt pathway. Mol Med Rep. 2017;16:5036–42.
Cardama GA, Gonzalez N, Maggio J, Lorenzano Menna P, Gomez DE. Rho GTPases as therapeutic targets in cancer. Int J Oncol. 2017;51:1025–34.
Zheng CC, Hu HF, Zhang QH, Xu W, He QY, Li B. Significance of integrin-linked kinase (ILK) in tumorigenesis and its potential implication as a biomarker and therapeutic target for human cancer. Am J Cancer Res. 2019;9:186–97.
André F, Ciruelos E, Rubovszky G, Campone M, Silbylle L, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929–40.
Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–57.
Acknowledgements
We thank Laura Tsujikawa for her help with IPA® data upload and analysis. The work has been funded by Zenith Epigenetics.
Author information
Authors and Affiliations
Contributions
RGP, CC, and OK conducted experiments, analyzed, and interpreted the data. OK and EvH conception and supervision of the study, wrote the paper. All authors corrected draft versions and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors are the employees and shareholders of Zenith Epigenetics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kharenko, O.A., Patel, R.G., Calosing, C. et al. Combination of ZEN-3694 with CDK4/6 inhibitors reverses acquired resistance to CDK4/6 inhibitors in ER-positive breast cancer. Cancer Gene Ther 29, 859–869 (2022). https://doi.org/10.1038/s41417-021-00375-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00375-9
This article is cited by
-
Bromodomain and extraterminal (BET) proteins: biological functions, diseases, and targeted therapy
Signal Transduction and Targeted Therapy (2023)
-
A review on the role of cyclin dependent kinases in cancers
Cancer Cell International (2022)