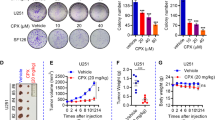
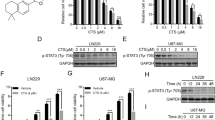
Abstract
A significant roadblock in treatment of GBM multiforme (GBM) is resistance to temozolomide (TMZ). In this study, we investigated whether I-BET151, a specific BET inhibitor, could sensitize GBM cells to TMZ. Our findings showed that the action of I-BET151 could augment the effect of TMZ on cancer cells U251 and U87 cells. In U251 cells, administration of I-BET151 increased the TMZ-induced apoptosis GBM cells. I-BET151 remarkably enhanced the activities of caspase-3. In addition, I-BET151 promoted TMZ-induced migration and invasion in GBM cells. Moreover, I-BET151 increased the amount of reactive oxygen species as well as superoxide anions with a decrease of activity of SOD and the anti-oxidative properties of GBM cells. I-BET151 also induced increased PUMA expression, which is required for the functions of I-BET151 and regulates the synergistic cytotoxic effects of i-BET151 and TMZ in GBM cells. I-BET151 with TMZ also showed synergistic cytotoxic effects in vivo. These point out to an approach to tackle GBM using TMZ along with BET inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
01 February 2019
All animal experiments were approved by the Animal Care and Use Committee (ACUC), Louisiana State University Health Science Center and not The People's Hospital of Liaoning Province as indicated in the original version of the Article. The PDF and HTML versions of the Article have been modified accordingly.
20 September 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41417-022-00536-4
References
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjhee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3–9.
Shergalis A, et al. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70:412–445.
Guan X, Hasan MN, Maniar S, Jia W, Sun D. Reactive astrocytes in glioblastoma multiforme. Mol Neurobiol. 2018;55:6927–6938.
Smrdel U, et al. Long-term survival in glioblastoma: methyl guanine methyl transferase (MGMT) promoter methylation as independent favourable prognostic factor. Radiol Oncol. 2016;50:394–401.
Lai RK, et al. Genome-wide methylation analyses in glioblastoma multiforme. PLoS ONE. 2014;9:e89376.
Fan CH, et al. methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876.
Valtorta S, et al. Metformin and temozolomide, a synergic option to overcome resistance in glioblastoma multiforme models. Oncotarget. 2017;8:113090–113104.
Johannessen TC, Bjerkvig R. Molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev AntiCancer Ther. 2012;12:635–642.
St-Coeur PD, Cormier M, LeBlanc VC, Morin PJ, Touaibia M. Effect of O6-substituted guanine analogs on O6-methylguanine DNA-methyltransferase expression and glioblastoma cells viability. Med Chem. 2016;13:28–39.
Anderson JC, et al. Kinomic exploration of temozolomide and radiation resistance in glioblastoma multiforme xenolines. Radiother Oncol. 2014;111:468–474.
Doroshow DB, Eder JP, LoRusso PM. BET inhibitors: a novel epigenetic approach. Ann Oncol. 2017;28:1776–1787.
Lochrin SE, Price DK, Figg WD. BET bromodomain inhibitors--a novel epigenetic approach in castration-resistant prostate cancer. Cancer Biol Ther. 2014;15:1583–1585.
Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem. 2012;287:36609–36616.
Loven J, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334.
Ocana A, Nieto-Jimenez C, Pandiella A. BET inhibitors as novel therapeutic agents in breast cancer. Oncotarget. 2017;8:71285–71291.
Wadhwa E, Nicolaides T. Bromodomain inhibitor review: bromodomain and extra-terminal family protein inhibitors as a potential new therapy in central nervous system tumors. Cureus. 2016;8:e620.
Zhang X., et al. Protein targeting chimeric molecules specific for bromodomain and extra-terminal motif family proteins are active against pre-clinical models of multiple myeloma. Leukemia. 2018;32:2224–2239.
Ott CJ, et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843–2852.
Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917.
Chaidos A, Caputo V, Karadimitris A. Inhibition of bromodomain and extra-terminal proteins (BET) as a potential therapeutic approach in haematological malignancies: emerging preclinical and clinical evidence. Ther Adv Hematol. 2015;6:128–141.
Knickelbein K, et al. Restoring PUMA induction overcomes KRAS-mediated resistance to anti-EGFR antibodies in colorectal cancer. Oncogene. 2018;37:4599–4610.
Huo L, Bai X, Wang Y, Wang M. Betulinic acid derivative B10 inhibits glioma cell proliferation through suppression of SIRT1, acetylation of FOXO3a and upregulation of Bim/PUMA. Biomed Pharmacother. 2017;92:347–355.
Xing SG, Zhang KJ, Qu JH, Ren YD, Luan Q. Propofol induces apoptosis of non-small cell lung cancer cells via ERK1/2-dependent upregulation of PUMA. Eur Rev Med Pharmacol Sci. 2018;22:4341–4349.
Ma J, Feng Y, Liu Y, Li X. PUMA and survivin are involved in the apoptosis of HepG2 cells induced by microcystin-LR via mitochondria-mediated pathway. Chemosphere. 2016;157:241–249.
Hikisz P, Kilianska ZMPUMA. a critical mediator of cell death--one decade on from its discovery. Cell Mol Biol Lett. 2012;17:646–669.
Lin L, Ding D, Jiang Y, Li Y, Li S. MEK inhibitors induce apoptosis via FoxO3a-dependent PUMA induction in colorectal cancer cells. Oncogenesis. 2018;7:67.
Sun L, et al. Ipatasertib, a novel Akt inhibitor, induces transcription factor FoxO3a and NF-kappaB directly regulates PUMA-dependent apoptosis. Cell Death Dis. 2018;9:911.
Yang S, et al. NVP-BKM120 inhibits colon cancer growth via FoxO3a-dependent PUMA induction. Oncotarget. 2017;8:83052–83062.
Zhang LN, Li JY, Xu W. A review of the role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug resistance of chronic lymphocytic leukemia. Cancer Gene Ther. 2013;20:1–7.
Chipuk JE, Green DR. PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle. 2009;8:2692–2696.
Tong J, et al. Mcl-1 phosphorylation without degradation mediates sensitivity to HDAC inhibitors by liberating BH3-only proteins. Cancer Res. 2018;78:4704–4715.
Tong J, Tan S, Zou F, Yu J, Zhang L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene. 2017;36:787–796.
Tong J, et al. FBW7-dependent Mcl-1 degradation mediates the anticancer effect of Hsp90 inhibitors. Mol Cancer Ther. 2017;16:1979–1988.
Tong J, et al. Mcl-1 degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res. 2017;77:2512–2521.
Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20:S2–S8.
Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O. Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy. Front Physiol. 2018;9:170.
Barciszewska AM, Gurda D, Glodowicz P, Nowak S, Naskret-Barciszewska MZ. A new epigenetic mechanism of temozolomide action in glioma cells. PLoS ONE. 2015;10:e0136669.
Zain J, Kaminetzky D, O’Connor OA. Emerging role of epigenetic therapies in cutaneous T-cell lymphomas. Expert Rev Hematol. 2010;3:187–203.
Sharma S, Gurudutta G. Epigenetic regulation of hematopoietic stem cells. Int J Stem Cells. 2016;9:36–43.
Segatto M, et al. Epigenetic targeting of bromodomain protein BRD4 counteracts cancer cachexia and prolongs survival. Nat Commun. 2017;8:1707.
Long J, et al. The BET bromodomain inhibitor I-BET151 acts downstream of smoothened protein to abrogate the growth of hedgehog protein-driven cancers. J Biol Chem. 2014;289:35494–35502.
Feng E, Sui C, Wang T, Sun G. Temozolomide with or without radiotherapy in patients with newly diagnosed glioblastoma multiforme: a meta-analysis. Eur Neurol. 2017;77:201–210.
Cheng Z, et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res. 2013;19:1748–1759.
Xu Y., Vakoc C. R. Targeting cancer cells with BET bromodomain inhibitors. Cold Spring Harb Perspect Med 2017;7:pii:a026674.
Aird F., Kandela I., Mantis C., Reproducibility project: Cancer B. Replication Study: BET bromodomain inhibition as a therapeutic strategy to target c-Myc. eLife. 2017;6:e21253.
da Motta LL, et al. The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. Oncogene. 2017;36:122–132.
Shamas-Din A, Kale J, Leber B, Andrews DW. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5:a008714.
Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80.
Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813:508–520.
Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:S71–p83.
Chen D, et al. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc Natl Acad Sci USA. 2018;115:3930–3935.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41417-022-00536-4
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Z., Yang, S., Zhao, H. et al. RETRACTED ARTICLE: BET inhibitor I-BET151 sensitizes GBM cells to temozolomide via PUMA induction. Cancer Gene Ther 27, 226–234 (2020). https://doi.org/10.1038/s41417-018-0068-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0068-4