Abstract
Background
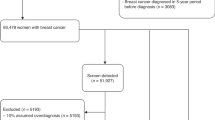
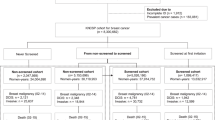
No studies are available in which changes over time in characteristics and prognosis of patients with interval breast cancers (ICs) and screen-detected breast cancers (SDCs) have been compared. The aim was to study these trends between 1995 and 2018.
Methods
All women with invasive SDCs (N = 4290) and ICs (N = 1352), diagnosed in a southern mammography screening region in the Netherlands, were included and followed until date of death or 31 December 2022.
Results
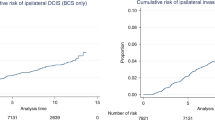
The 5-year overall survival rate of women with SDCs increased from 91.4% for those diagnosed in 1995–1999 to 95.0% for those diagnosed in 2013–2018 (P < 0.001), and from 74.8 to 91.6% (P < 0.001) in the same periods for those with ICs. A similar trend was observed for the 10-year survival rates. After adjustment for changes in tumour characteristics, the hazard ratio (HR) for overall survival was 0.47 (95% confidence interval (CI): 0.38–0.59) for women with SDCs diagnosed in the period 2013–2018, compared to the women diagnosed in the period 1995–1999. For the women with ICs this HR was 0.27 (95% CI: 0.19–0.40).
Conclusion
The prognosis of women with ICs has improved rapidly since 1995 and is now almost similar to that of women with SDCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data supporting the findings in this study are presented in the manuscript and the supplementary information, and additional raw data can be made available by the corresponding author upon reasonable request.
References
Peairs KS, Choi Y, Stewart RW. Screening for breast cancer. Semin Oncol. 2017;44:60–72.
IKNL. NKR Cijfers. Available from: https://iknl.nl/nkr-cijfers. Accessed on: 12-03-2022.
Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA. 2015;314:1615–34.
Zielonke N, Gini A, Jansen EEL, Anttila A, Segnan N, Ponti A, et al. Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: a systematic review. Eur J Cancer. 2020;127:191–206.
De Munck L, De Bock GH, Otter R, Reiding D, Broeders MJM, Willemse PHB, et al. Digital vs screen-film mammography in population-based breast cancer screening: performance indicators and tumour characteristics of screen-detected and interval cancers. Br J Cancer. 2016;115:517.
Van Luijt PA, Fracheboud J, Heijnsdijk EAM, Den Heeten GJ, De Koning HJ. Nation-wide data on screening performance during the transition to digital mammography: Observations in 6 million screens. Eur J Cancer. 2013;49:3517–25.
Nederend J, Duijm LEM, Louwman MWJ, Groenewoud JH, Donkers-Van Rossum AB, Voogd AC. Impact of transition from analog screening mammography to digital screening mammography on screening outcome in The Netherlands: a population-based study. Ann Oncol. 2012;23:3098–103.
van der Meer DJ, Kramer I, van Maaren MC, van Diest PJ, Linn SC, Maduro JH, et al. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. Int J Cancer. 2021;148:2289–303.
Vugts G, Maaskant-Braat AJG, Nieuwenhuijzen GAP, Roumen RMH, Luiten EJT, Voogd AC. Patterns of care in the administration of neo-adjuvant chemotherapy for breast cancer. a population-based study. Breast J. 2016;22:316–21.
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl J Med. 2005;353:1784–92.
Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203–10.
Grassmann F, He W, Eriksson M, Gabrielson M, Hall P, Czene K. Interval breast cancer is associated with other types of tumors. Nat Commun. 2019;10:4648.
Timmermans L, De Brabander I, Van Damme N, Bleyen L, Martens P, Van Herck K, et al. Tumour characteristics of screen-detected and interval cancers in the flemish breast cancer screening programme: a mammographic breast density study. Maturitas. 2022;158:55–60.
Ernst MF, Voogd AC, Coebergh JWW, Roukema JA. Breast carcinoma diagnosis, treatment, and prognosis before and after the introduction of mass mammographic screening. Cancer. 2004;100:1337–44.
Rijksinstituut voor Volksgezonheid en Milieu. Bevolkingsonderzoek borstkanker. Available from: https://www.rivm.nl/bevolkingsonderzoek-borstkanker. Accessed on: 12-06-2022.
Weber RJP, van Bommel RMG, Louwman MW, Nederend J, Voogd AC, Jansen FH, et al. Characteristics and prognosis of interval cancers after biennial screen-film or full-field digital screening mammography. Breast Cancer Res Treat. 2016;158:471–83.
Rakha EA, El-Sayed ME, Lee AHS, Elston CW, Grainge MJ, Hodi Z, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26:3153–8.
American College of Radiology. Illustrated Breast Imaging Reporting and Data System (BI-RADS). 3rd edn. Reston, VA, USA, 1998.
American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS). 4th edn. Reston, VA, USA, 2003.
American College of Radiology, BI-RADS Committee. ACR BI-RADS atlas: breast imaging reporting and data system. 5th edn. Reston, VA, USA, 2013.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
McNeish D. Missing data methods for arbitrary missingness with small samples. J Appl Stat. 2016;44:24–39.
Bellio G, Marion R, Giudici F, Kus S, Tonutti M, Zanconati F, et al. Interval breast cancer versus screen-detected cancer: comparison of clinicopathologic characteristics in a single-center analysis. Clin Breast Cancer. 2017;17:564–71.
Domingo L, Blanch J, Servitja S, Corominas JM, Murta-Nascimento C, Rueda A, et al. Aggressiveness features and outcomes of true interval cancers: comparison between screen-detected and symptom-detected cancers. Eur J Cancer Prev. 2013;22:21–8.
Weber RJP, van Bommel RMG, Setz-Pels W, Voogd AC, Klompenhouwer EG, Louwman MW, et al. Type and extent of surgery for screen-detected and interval cancers at blinded versus nonblinded double-reading in a population-based screening mammography program. Ann Surg Oncol. 2016;23:3822–30.
Niraula S, Biswanger N, Hu P, Lambert P, Decker K. Incidence, characteristics, and outcomes of interval breast cancers compared with screening-detected breast cancers. JAMA Netw Open. 2020;3:e2018179.
Hofvind S, Holen Å, Román M, Sebuødegård S, Puig-Vives M, Akslen L. Mode of detection: an independent prognostic factor for women with breast cancer. J Med Screen. 2016;23:89–97.
De Munck L, Schaapveld M, Siesling S, Wesseling J, Voogd AC, Tjan-Heijnen VCG, et al. Implementation of trastuzumab in conjunction with adjuvant chemotherapy in the treatment of non-metastatic breast cancer in the Netherlands. Breast Cancer Res Treat. 2011;129:229–33.
Beek MA, Verheuvel NC, Luiten EJT, Klompenhouwer EG, Rutten HJT, Roumen RMH, et al. Two decades of axillary management in breast cancer. Br J Surg. 2015;102:1658–64.
Blok EJ, Kroep JR, Kranenbarg EMK, Duijm-De Carpentier M, Putter H, Van Den Bosch J, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006-05). J Natl Cancer Inst. 2018;110:40–8.
Derks MGM, Bastiaannet E, van de Water W, de Glas NA, Seynaeve C, Putter H, et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Cancer. 2018;99:1–8.
Long H, Brooks JM, Harvie M, Maxwell A, French DP. How do women experience a false-positive test result from breast screening? A systematic review and thematic synthesis of qualitative studies. Br J Cancer. 2019;121:351–8.
Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 U.S. preventive services task force recommendation. Ann Intern Med. 2016;164:256–67.
Bolejko A, Zackrisson S, Hagell P, Wann-Hansson C. A roller coaster of emotions and sense-coping with the perceived psychosocial consequences of a false-positive screening mammography. J Clin Nurs. 2014;23:2053–62.
Dejean D, Krahn H, Giacomini M, Zierler A, Dhalla I, Sikich N, et al. Women’s experiences of inaccurate breast cancer screening results: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2016;16:1.
Houssami N, Zackrisson S, Blazek K, Hunter K, Bernardi D, Lång K, et al. Meta-analysis of prospective studies evaluating breast cancer detection and interval cancer rates for digital breast tomosynthesis versus mammography population screening. Eur J Cancer (Oxf, Engl: 1990). 2021;148:14–23.
Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091–102.
Allweis TM, Hermann N, Berenstein-Molho R, Guindy M. Personalized screening for breast cancer: rationale, present practices, and future directions. Ann Surg Oncol. 2021;28:4306–17.
Saccarelli CR, Bitencourt AGV, Morris EA. Is it the era for personalized screening? Radiol Clin North Am. 2021;59:129–38.
Geertse TD, van der Waal D, Vreuls W, Tetteroo E, Duijm LEM, Pijnappel RM, et al. The dilemma of recalling well-circumscribed masses in a screening population: A narrative literature review and exploration of Dutch screening practice. Breast (Edinb, Scotl). 2023;69:431–40.
Mook S, Van ’T Veer LJ, Rutgers EJ, Ravdin PM, Van De Velde AO, Van Leeuwen FE, et al. Independent prognostic value of screen detection in invasive breast cancer. J Natl Cancer Inst. 2011;103:585–97.
Duffy SW, Tabár L, Yen AMF, Dean PB, Smith RA, Jonsson H, et al. Beneficial effect of consecutive screening mammography examinations on mortality from breast cancer: a prospective study. Radiology. 2021;299:541–7.
Author information
Authors and Affiliations
Contributions
Study design: LEMD and ACV. Performed the research and collected data: LEMD, DEV and ACV. Analyzed the data: DEV, LEMD and ACV. Manuscript drafting: DEV, ACV and LEM. Provided discussion, critical feedback and manuscript editing: ACV, LEM, MJCS, RJS, VCGTH, WV, LJAS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
ten Velde, D.E., Duijm, L.E.M., van der Sangen, M.J.C. et al. Long-term trends in incidence, characteristics and prognosis of screen-detected and interval cancers in women participating in the Dutch breast cancer screening programme. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02633-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02633-7