Abstract
Background
This study evaluated the non-inferiority of dexamethasone (DEX) on day 1, with sparing on days 2–4 in cisplatin-based chemotherapy.
Methods
Patients with malignant solid tumors who were treated with cisplatin (≥50 mg/m²) were randomly assigned (1:1) to receive either DEX on days 1–4 (Arm D4) or DEX on day 1 (Arm D1) plus palonosetron, NK-1 RA, and olanzapine (5 mg). The primary endpoint was complete response (CR) during the delayed (24–120 h) phase. The non-inferiority margin was set at −15%.
Results
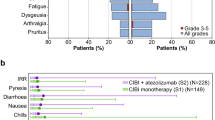
A total of 281 patients were enrolled, 278 of whom were randomly assigned to Arm D4 (n = 139) or Arm D1 (n = 139). In 274 patients were included in the efficacy analysis, the rates of delayed CR in Arms D4 and D1 were 79.7% and 75.0%, respectively (risk difference −4.1%; 95% CI –14.1%–6.0%, P = 0.023). However, patients in Arm D1 had significantly lower total control rates during the delayed and overall phases, and more frequent nausea and appetite loss. There were no significant between-arm differences in the quality of life.
Conclusion
DEX-sparing is an alternative option for patients receiving cisplatin; however, this revised administration schedule should be applied on an individual basis after a comprehensive evaluation.
Clinical Trials Registry number
UMIN000032269
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data were generated by the authors and are available on request.
References
Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Eng J Med. 2016;374:1356–67.
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15:103–9.
Aogi K, Takeuchi H, Saeki T, Aiba K, Tamura K, Iino K, et al. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol. 2021;26:1–17.
Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:242–9.
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Eng J Med. 2016;375:134–42.
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3240–61.
Razvi Y, Chan S, McFarlane T, McKenzie E, Zaki P, DeAngelis C, et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer. 2019;27:87–95.
Grunberg S. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol. 2007;18:233–40.
Vardy J, Chiew K, Galica J, Pond G, Tannock I. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006;94:1011–5.
Jeong Y, Han HS, Lee HD, Yang J, Jeong J, Choi MK, et al. A pilot study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Ttreat. 2016;48:1429–37.
Nakamura M, Ishiguro A, Muranaka T, Fukushima H, Yuki S, Ono K, et al. A prospective observational study on effect of short‐term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO‐01). Oncologist. 2017;22:592–600.
Celio L, Bonizzoni E, Zattarin E, Codega P, De Braud F, Aapro M. Impact of dexamethasone-sparing regimens on delayed nausea caused by moderately or highly emetogenic chemotherapy: a meta-analysis of randomised evidence. BMC Cancer. 2019;19:1–13.
Ito Y, Tsuda T, Minatogawa H, Kano S, Sakamaki K, Ando M, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol. 2018;36:1000–6.
Yeo W, Lau TK, Li L, Lai KT, Pang E, Cheung M, et al. A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast. 2020;50:30–38.
Minatogawa H, Izawa N, Kawaguchi T, Miyaji T, Shimomura K, Kazunori H, et al. Study protocol for SPARED trial: randomised non-inferiority phase III trial comparing dexamethasone on day 1 with dexamethasone on days 1–4, combined with neurokinin-1 receptor antagonist, palonosetron and olanzapine (5 mg) in patients receiving cisplatin-based chemotherapy. BMJ Open. 2020;10:e041737.
Miyaji T, Iioka Y, Kuroda Y, Yamamoto D, Iwase S, Goto Y, et al. Japanese translation and linguistic validation of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Patient Rep Outcomes. 2017;1:1–10.
Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9:188–95.
Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, et al. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol. 2018;23:382–8.
Nakashima K, Murakami H, Yokoyama K, Omori S, Wakuda K, Ono A, et al. A phase II study of palonosetron, aprepitant, dexamethasone and olanzapine for the prevention of cisplatin-based chemotherapy-induced nausea and vomiting in patients with thoracic malignancy. Jpn J Clin Oncol. 2017;47:840–3.
Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28:1260–7.
Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, et al. Effects of systemically administered hydrocortisone on the human immunome. Sci Rep. 2016;6:1–15.
Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–63.
Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med. 2016;13:e1002024.
Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8.
Europiean Sociaty for Medical Oncology: Supportive Care Strategies During the COVID-19 Pandemic. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/supportive-care-in-the-covid-19-era
Grant RC, Rotstein C, Liu G, Forbes L, Vu K, Lee R, et al. Reducing dexamethasone antiemetic prophylaxis during the COVID-19 pandemic: recommendations from Ontario, Canada. Support Care Cancer. 2020;28:5031–6.
Schotte A, Janssen P, Gommeren W, Luyten W, Van Gompel P, Lesage A, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73.
Nikbakhsh N, Sadeghi MV, Ramzani E, Moudi S, Bijani A, Yousefi R, et al. Efficacy of olanzapine in symptom relief and quality of life in gastric cancer patients receiving chemotherapy. J Res Med Sci. 2016;21:88.
Celio L, Cortinovis D, Cogoni AA, Cavanna L, Martelli O, Carnio S, et al. Dexamethasone-sparing regimens with oral netupitant and palonosetron for the prevention of emesis caused by high-dose cisplatin: a randomized noninferiority study. Oncologist. 2021;26:e1854–e61.
Hata A, Okamoto I, Inui N, Okada M, Morise M, Akiyoshi K, et al. Randomized, double-blind, phase III study of fosnetupitant versus fosaprepitant for prevention of highly emetogenic chemotherapy-induced nausea and vomiting: CONSOLE. J Clin Oncol. 2022;40:180–8.
Acknowledgements
The authors thank all the patients and their families, investigators, and institutions involved in this study. We are also grateful to Tomoe Mashiko for data management, Shunsuke Oyamada for data analysis, and Yoshiki Horie for helpful discussions. This study is supported by Japan Agency for Medical Research and Development (AMED) grant number 21ck0106501h0003. AMED had and will have no involvement in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript. The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This study was funded by the Japan Agency for Medical Research and Development (grant number 21ck0106501h0003).
Author information
Authors and Affiliations
Contributions
HM, NI and TEN contributed to the trial conception and are the principal investigators. HM, NI, TM, TK, TY, and TEN participated in the design of the study. TY did the statistical analysis. TM and TK contributed to data management. HM, NI, statistician (TY), and TEN had full access to the data, conducted data analysis and interpretation, and take responsibility for the integrity of the data and adherence to the study protocol. HM and NI contributed to the initial draft of the manuscript and are responsible for the decision to submit the manuscript for publication. All authors contributed to data collection and interpretation, and revision of the manuscript for important content.
Corresponding author
Ethics declarations
Competing interests
HA reports personal fees from Ono, Bristol-Myers Squibb, Daiichi-Sankyo, Takeda, Chugai, Eisai and Delta-Fly Pharma. HI reports grants from Daiichi Sankyo Co., Ltd., Nippon Kayaku Co., Ltd., Chugai,,, Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Asahi Kasei Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd.; and reports personal fees from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Astellas Pharma Co., Ltd., Eli Lilly and Company, Daiichi Sankyo Co., Ltd., AstraZeneca plc, Nippon Kayaku Co., Ltd., Ono, Pharmaceutical Co., Ltd. and Nippon Boehringer Ingelheim Co., Ltd. TM reports grants from AC Medical, A2 Healthcare, CAC Croit Corporation, Japan Tobacco Inc, Japan Media Corporation, Medidata Solutions, Inc, Ono Pharmaceutical, FMD K&L Japan, Intellim, Welby, Nipro Corporation, New Age Trading, NOBORI Ltd. And Medrio; and reports personal fees from AYUMI, Impute, Pfizer, Welby, EISAI, Merc and Takeda. KH reports grants from Pfizer. JE reports grants from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nippon, Boehringer Ingelheim Co., Ltd., Kyorin Holdings, Inc. and Nippon Kayaku Co., Ltd.; and reports personal fees from AstraZeneca plc, Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., Takeda Pharmaceutical Co., Ltd., Novartis Pharma K.K. and Daiichi Sankyo Co., Ltd. NF reports personal fees from Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim Japan, Chugai, Bristol Myers Squibb, Taiho, Pfizer Japan and Novartis. TY reports grants from AC MEDICAL INC., A2 Healthcare Corporation, EP Croit Co., Ltd., ClinChoice., Japan Tobacco Inc., Japan Media Corporation, Medidata Solutions, Inc., ONO PHARMACEUTICAL CO., LTD., Kyowa Kirin Co., Ltd., TSUMURA & CO., DAIICHI, SANKYO COMPANY, LIMITED., Otsuka Pharmaceutical Co., Ltd. Eisai Co., Ltd., ASAHI, INTECC CO., LTD., 3H Clinical Trial Inc., Medrio, Inc., NIPRO CORPORATION, Intellim Corporation, Welby Inc., 3H Medi Solution Inc., NIPRO CORPORATION, Baseconnect Inc., Nobori Ltd., Puravida Technologies LLC. and Hemp Kitchen Inc.; and reports personal fees from EPS Corporation, Japan Tobacco Inc., Medidata Solutions, Inc., ONO PHARMACEUTICAL CO., LTD., Kowa Company, Ltd., CHUGAI PHARMACEUTICAL CO. LTD., TSUMURA & CO., DAIICHI SANKYO COMPANY, LIMITED., Eisai Co., Ltd., ASAHI INTECC CO., LTD., ASAHI KASEI PHARMA CORPORATION, 3H Clinical Trial Inc. Intellim Corporation, Takeda, AstraZeneca, SONIRE Therapeutics Inc., SEIKAGAKU CORPORATION, Merck & Co., Inc. and NIPRO CORPORATION. TEN reports grants from Sumitomo Dainippon Pharma Co., Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Takeda Pharmaceutical Co., Chugai Pharmaceutical Co., Sanofi K.K., Nippon Kayaku Co., MSD K.K. and Eli Lilly Japan K.K.; and reports personal fees from Thyas Co. Ltd., Rebirthel Co., Ltd., Japan Clinical Research Operations, Sumitomo Dainippon Pharma Co., Boehringer Ingelheim, Bristol-Myers Squibb, Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Amgen, Takeda Pharmaceutical Co., Chugai Pharmaceutical Co., Sanofi K.K., Novartis Japan, Nippon Kayaku Co., MSD K.K., Eli Lilly Japan K.K., Bayer Yakuhin, Pfizer Japan Inc., Daiichi, Sankyo Co., Yakult Honsha Co., Nipro Co, Merck Serono Co., AstraZeneca, IQVIA and GlaxoSmithKline. All other authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board at each site. The study was registered with the University Hospital Medical Information Network, Clinical Trials Registry number UMIN000032269. All patients included in this study provided written, informed consent.
Consent for publication
All patients provided written, informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minatogawa, H., Izawa, N., Shimomura, K. et al. Dexamethasone-sparing on days 2–4 with combined palonosetron, neurokinin-1 receptor antagonist, and olanzapine in cisplatin: a randomized phase III trial (SPARED Trial). Br J Cancer 130, 224–232 (2024). https://doi.org/10.1038/s41416-023-02493-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02493-7
This article is cited by
-
Reply to L. Celio et al.
British Journal of Cancer (2024)
-
Comment on “Dexamethasone-sparing on days 2–4 with combined palonosteron, neurokinin-1 receptor antagonist, and olanzapine in cisplatin: a randomized phase III trial (SPARED Trial)”
British Journal of Cancer (2024)
-
Efficacy and safety of netupitant/palonosetron in preventing nausea and vomiting in diffuse large B cell lymphoma patients undergoing R–CHOP chemotherapy
Scientific Reports (2024)