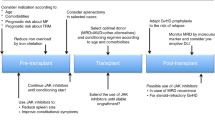
Abstract
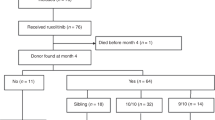
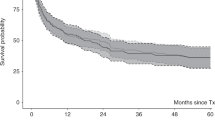
In this SFGM-TC registry study, we report the results after stem cell transplantation (HSCT) in 305 myelofibrosis patients, in order to determine potential risk factors associated with outcomes, especially regarding previous treatment with ruxolitinib. A total of 102 patients were transplanted from an HLA-matched-sibling donor (MSD), and 143 patients received ruxolitinib. In contrast with previous studies, our results showed significantly worse outcomes for ruxolitinib patients regarding overall survival (OS) and non-relapse mortality (NRM), especially in the context of unrelated donors (URD). When exploring reasons for potential confounders regarding the ruxolitinib effect, an interaction between the type of donor and the use of ATG was found, therefore subsequent analyses were performed separately for each type of donor. Multivariable analyses did not confirm a significant negative impact of ruxolitinib in transplantation outcomes. In the setting of URD, only age and Fludarabine-Melphalan (FM) conditioning were associated with increased NRM. For MSD, only Karnoksfy <70% was associated with reduced OS. However, a propensity score analysis showed that ruxolitinib had a negative impact on OS but only in non-responding patients, consistent with previous data. To conclude, with all the precautions due to confounders and bias, ruxolitinib itself does not appear to increase mortality after HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available via SFGM-TC. These data are available upon reasonable request and with the permission of SFGM-TC.
References
Passamonti F, Mora B. Myelofibrosis. Blood. 2023;141:1954–70. https://doi.org/10.1182/blood.2022017423
Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:801–21. https://doi.org/10.1002/ajh.26857
Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105:973–7. https://doi.org/10.1182/blood-2004-07-2864
Kröger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. Published online August 21, 2015. https://doi.org/10.1038/leu.2015.233
Gupta V, Malone AK, Hari PN, Ahn KW, Hu ZH, Gale RP, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2014;20:89–97. https://doi.org/10.1016/j.bbmt.2013.10.018
Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–70. https://doi.org/10.1182/blood-2009-07-234880
Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–91. https://doi.org/10.1182/blood-2014-04-572545
Hernández-Boluda JC, Pereira A, Alvarez-Larran A, Martín AA, Benzaquen A, Aguirre L, et al. Predicting survival after allogeneic hematopoietic cell transplantation in myelofibrosis: performance of the Myelofibrosis Transplant Scoring System (MTSS) and Development of A New Prognostic Model. Biol Blood Marrow Transpl. 2020;26:2237–44. https://doi.org/10.1016/j.bbmt.2020.07.022
Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transpl. 2010;45:458–63. https://doi.org/10.1038/bmt.2009.188
Robin M, Espérou H, de Latour RP, Petropoulou AD, Xhaard A, Ribaud P, et al. Splenectomy after allogeneic haematopoietic stem cell transplantation in patients with primary myelofibrosis. Br J Haematol. 2010;150:721–4. https://doi.org/10.1111/j.1365-2141.2010.08276.x
Robin M, Tabrizi R, Mohty M, Furst S, Michallet M, Bay JO, et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Br J Haematol. 2011;152:331–9. https://doi.org/10.1111/j.1365-2141.2010.08417.x
Robin M, Porcher R, Orvain C, Bay JO, Barraco F, Huynh A, et al. Ruxolitinib before allogeneic hematopoietic transplantation in patients with myelofibrosis on behalf SFGM-TC and FIM groups. Bone Marrow Transpl. 2021;56:1888–99. https://doi.org/10.1038/s41409-021-01252-7
Kröger N, Sbianchi G, Sirait T, Wolschke C, Beelen D, Passweg J, et al. Impact of prior JAK-inhibitor therapy with ruxolitinib on outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis: a study of the CMWP of EBMT. Leukemia. 2021;35:3551–60. https://doi.org/10.1038/s41375-021-01276-4
Jain T, Kunze KL, Temkit M, Partain DK, Patnaik MS, Slack JL, et al. Comparison of reduced intensity conditioning regimens used in patients undergoing hematopoietic stem cell transplantation for myelofibrosis. Bone Marrow Transpl. 2019;54:204–11. https://doi.org/10.1038/s41409-018-0226-1
Robin M, Porcher R, Wolschke C, Sicre de Fontbrune F, Alchalby H, Christopeit M, et al. Outcome after transplantation according to reduced-intensity conditioning regimen in patients undergoing transplantation for myelofibrosis. Biol Blood Marrow Transpl. 2016;22:1206–11. https://doi.org/10.1016/j.bbmt.2016.02.019
Murthy GSG, Kim S, Estrada-Merly N, Abid MB, Aljurf M, Assal A, et al. Association between the choice of the conditioning regimen and outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica. 2023;108:1900–8. https://doi.org/10.3324/haematol.2022.281958
Robin M, Chevret S, Koster L, Wolschke C, Yakoub-Agha I, Bourhis JH, et al. Antilymphocyte Globulin for matched sibling donor transplantation in patients with myelofibrosis. Haematologica. Published online January 17, 2019. https://doi.org/10.3324/haematol.2018.201400
Gagelmann N, Ditschkowski M, Bogdanov R, Bredin S, Robin M, Cassinat B, et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood. 2019;133:2233–42. https://doi.org/10.1182/blood-2018-12-890889
Jain T, Tsai HL, DeZern AE, Gondek LP, Elmariah H, Bolaños-Meade J, et al. Post-transplantation cyclophosphamide-based graft- versus-host disease prophylaxis with nonmyeloablative conditioning for blood or marrow transplantation for myelofibrosis. Transpl Cell Ther. 2022;28:259.e1–259.e11. https://doi.org/10.1016/j.jtct.2022.02.004
Kunte S, Rybicki L, Viswabandya A, Tamari R, Bashey A, Keyzner A, et al. Allogeneic blood or marrow transplantation with haploidentical donor and post-transplantation cyclophosphamide in patients with myelofibrosis: a multicenter study. Leukemia. 2022;36:856–64. https://doi.org/10.1038/s41375-021-01449-1
Acknowledgements
The authors acknowledge all centers, investigators, data managers, patients, and their families for their participation in this study, and all the SFGM-TC members.
Author information
Authors and Affiliations
Contributions
MR and SV wrote the manuscript. SC performed the statistical analysis. XP, AC, PC, JHB, EF, SC, DB, JOB, ML, RD, RDu, PCe, MTR, YB, and SN recruited the patients. NR has done data management. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests in relationship with the current study. MR: research support by Novartis, MEDAC, NEOVII, ASTEX, ABBVIE. RD: research funding from Ligue contre le Cancer, Arthur Sachs, Monahan Foundation, Servier Foundation, Philippe Foundation, DCP AP-HP, honoraria from Novartis and Takeda, and non-financial support from Kite Pharma/Gilead, all outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Villar, S., Chevret, S., Poire, X. et al. Transplantation for myelofibrosis patients in the ruxolitinib era: a registry study from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02268-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02268-5