Abstract
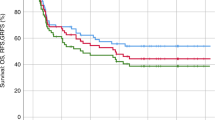
To define the efficacy of a single dose of 375 mg/m2 rituximab for DSA-positive patients with 2000 ≤ MFI < 10,000, we enrolled a prospective clinical cohort including patients with positive DSA treated with rituximab (n = 55, cohort A), a matched-pair cohort including cases with negative DSA (n = 110, cohort B) and a historical cohort including subjects with 2000 ≤ MFI < 10,000 without receiving any treatment for DSA (n = 22, cohort C). The incidences of primary poor graft function (PGF) in cohort A and cohort B were 5% and 1% (P = 0.076), respectively, both of which were lower than that in cohort C (27%, P < 0.001, for all). Rituximab was associated with a reduced incidence of primary PGF (HR 0.200, P = 0.023). The 3-year nonrelapse mortality of patients in cohort A and cohort B were 23% and 24%, respectively, both of which were lower than that in the cohort C (37%), although no statistical significance was observed. These results led to a low 3-year overall survival in patients in the cohort C (58%) compared with those in the cohort A (71%) and the cohort B (73%). We suggest that a single dose of rituximab could be effectively used to prevent the onset of primary PGF. The prospective cohort of this study is registered at http://www.chictr.org.cn/ChiCTR-OPC-15006672.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62. https://doi.org/10.1182/blood-2015-02-627786
Schmitz N, Lenz G, Stelljes M. Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood. 2018;132:245–53. https://doi.org/10.1182/blood-2018-01-791335
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6. https://doi.org/10.1200/JCO.2012.44.3523
Martinez C, Gayoso J, Canals C, Finel H, Peggs K, Dominietto A, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the lymphoma working party of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2017;35:3425–32. https://doi.org/10.1200/JCO.2017.72.6869
Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379:2330–41. https://doi.org/10.1056/NEJMoa1808777
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Clin Cancer Res. 2016;22:3467–76. https://doi.org/10.1158/1078-0432.CCR-15-2335
Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. https://doi.org/10.1186/s13045-015-0182-9
Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–24. https://doi.org/10.1097/TP.0b013e3181b9d710
Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118:5957–64. https://doi.org/10.1182/blood-2011-06-362111
Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118:6691–7. https://doi.org/10.1182/blood-2011-05-355263
Gladstone DE, Bettinotti MP. HLA donor-specific antibodies in allogeneic hematopoietic stem cell transplantation: challenges and opportunities. Hematol Am Soc Hematol Educ Program. 2017;2017:645–50. https://doi.org/10.1182/asheducation-2017.1.645
Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47:508–15. https://doi.org/10.1038/bmt.2011.131
Delbos F, Barhoumi W, Cabanne L, Beckerich F, Robin C, Redjoul R, et al. Donor immunization against human leukocyte class II antigens is a risk factor for graft-versus-host disease. Biol Blood Marrow Transplant. 2016;22:292–9. https://doi.org/10.1016/j.bbmt.2015.09.027
Yamamoto H, Uchida N, Matsuno N, Ota H, Kageyama K, Wada S, et al. Anti-HLA antibodies other than against HLA-A, -B, -DRB1 adversely affect engraftment and nonrelapse mortality in HLA-mismatched single cord blood transplantation: possible implications of unrecognized donor-specific antibodies. Biol Blood Marrow Transplant. 2014;20:1634–40. https://doi.org/10.1016/j.bbmt.2014.06.024
Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Societe Francophone d’Histocompatibilite et d’Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013;98:1154–60. https://doi.org/10.3324/haematol.2012.077685
Kong Y. Poor graft function after allogeneic hematopoietic stem cell transplantation-an old complication with new insights. Semin Hematol. 2019;56:215–20. https://doi.org/10.1053/j.seminhematol.2018.08.004
Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:521–34. https://doi.org/10.1038/s41409-017-0062-8
Choe H, Gergis U, Hsu J, Phillips A, Shore T, Christos P, et al. Bortezomib and immune globulin have limited effects on donor-specific HLA antibodies in haploidentical cord blood stem cell transplantation: detrimental effect of persistent haploidentical donor-specific HLA antibodies. Biol Blood Marrow Transplant. 2019;25:e60–4. https://doi.org/10.1016/j.bbmt.2018.10.018
Garnier A, Delbos F, Guillaume T, Peterlin P, Le Bourgeois A, Bene MC, et al. Rituximab for second desensitization in patients with rebound of donor-specific anti-HLA antibodies before T-replete haplo-transplant using high-dose post-transplant cyclophosphamide. Bone Marrow Transplant. 2018;53:1044–7. https://doi.org/10.1038/s41409-018-0107-7
Wilk CM, Fischer JC, Schieren G, Rox JM, Haas R, Rump LC, et al. Treatment of donor-specific antibody-mediated graft rejection by immunochemotherapy, third-party DLI, plasmapheresis and immunoadsorption. Bone Marrow Transplant. 2015;50:613–4. https://doi.org/10.1038/bmt.2014.321
Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–51. https://doi.org/10.1056/NEJMoa0707894
Zhou H, Xu M, Qin P, Zhang HY, Yuan CL, Zhao HG, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125:1541–7. https://doi.org/10.1182/blood-2014-06-581868
Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017;129:2971–9. https://doi.org/10.1182/blood-2016-11-693689
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019. https://doi.org/10.1136/annrheumdis-2019-215089
Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 2008;8:2607–17. https://doi.org/10.1111/j.1600-6143.2008.02411.x
van den Hoogen MW, Kamburova EG, Baas MC, Steenbergen EJ, Florquin S, MK HJ, et al. Rituximab as induction therapy after renal transplantation: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant. 2015;15:407–16. https://doi.org/10.1111/ajt.13052
Minakawa K, Ohto H, Yasuda H, Saito S, Kawabata K, Ogawa K, et al. Efficacy of D- red blood cell transfusion and rituximab therapy in autoimmune hemolytic anemia with anti-D and panreactive autoantibodies arising after hematopoietic stem cell transplant. Transfusion. 2018;58:1606–10. https://doi.org/10.1111/trf.14634
La Rocca U, Perrone MP, Piciocchi A, Cinti P, Barberi W, Gozzer M, et al. Anti-HLA donor-specific antibodies in allogeneic stem cell transplantation: management and desensitization protocol. Bone Marrow Transplant. 2019. https://doi.org/10.1038/s41409-019-0497-1
Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24. https://doi.org/10.1038/nrclinonc.2015.128
Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79:1507–15. https://doi.org/10.1097/01.tp.0000164159.20075.16. e-pub ahead of print 2005/06/09
Lv M, Zhai SZ, Wang Y, Xu LP, Zhang XH, Chen H, et al. Class I and II human leukocyte antibodies in pediatric haploidentical allograft candidates: prevalence and risk factors. Bone Marrow Transplant. 2019;54:1287–94. https://doi.org/10.1038/s41409-018-0427-7. e-pub ahead of print 2019/01/19
Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol. 2016;34:1855–63. https://doi.org/10.1200/JCO.2015.63.8817
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119:978–85. https://doi.org/10.1002/cncr.27761
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–26.
Hiwase SD, Dyson PG, To LB, Lewis ID. Cotransplantation of placental mesenchymal stromal cells enhances single and double cord blood engraftment in nonobese diabetic/severe combined immune deficient mice. Stem Cells. 2009;27:2293–2300. https://doi.org/10.1002/stem.157
Wu KH, Sheu JN, Wu HP, Tsai C, Sieber M, Peng CT, et al. Cotransplantation of umbilical cord-derived mesenchymal stem cells promote hematopoietic engraftment in cord blood transplantation: a pilot study. Transplantation. 2013;95:773–7. https://doi.org/10.1097/TP.0b013e31827a93dd
Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014;23:1087–98. https://doi.org/10.3727/096368912X661319
Scrucca L1, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–87.
Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1392–8. https://doi.org/10.1016/j.bbmt.2015.05.001
Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–45. https://doi.org/10.1182/blood-2011-08-372508
Chen J, Wang RX, Chen F, Sun AN, Qiu HY, Jin ZM, et al. Combination of a haploidentical SCT with an unrelated cord blood unit: a single-arm prospective study. Bone Marrow Transplant. 2014;49:206–11. https://doi.org/10.1038/bmt.2013.154
Saadi G, Fadel F, El Ansary M, El-Hamid SA. Mesenchymal stem cell transfusion for desensitization of positive lymphocyte cross-match before kidney transplantation: outcome of 3 cases. Cell Prolif. 2013;46:121–6. https://doi.org/10.1111/cpr.12012
Bramanti S, Calafiore V, Longhi E, Mariotti J, Crespiatico L, Sarina B, et al. Donor-specific anti-HLA antibodies in haploidentical stem cell transplantation with post-transplantation cyclophosphamide: risk of graft failure, poor graft function, and impact on outcomes. Biol Blood Marrow Transplant. 2019;25:1395–406. https://doi.org/10.1016/j.bbmt.2019.02.020
Acknowledgements
This work was supported (in part) by the National Key Research and Development Program of China (Grant No. 2017YFA0104500), the Beijing Municipal Science and Technology Commission (Grant No. Z181100009618032), the National Natural Science Foundation of China (Grant No. 81470342), and the Key Program of National Natural Science Foundation of China (Grant No. 81230013).
Author information
Authors and Affiliations
Contributions
Contribution: X-JH designed the study. Y-JC collected the data. L-PX, Y-JC, and X-JH analyzed the data and drafted the paper. All the authors contributed to data interpretation, paper preparation, and approval of the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, YJ., Xu, LP., Wang, Y. et al. Rituximab for desensitization during HLA-mismatched stem cell transplantation in patients with a positive donor-specific anti-HLA antibody. Bone Marrow Transplant 55, 1326–1336 (2020). https://doi.org/10.1038/s41409-020-0928-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0928-z
This article is cited by
-
Acute mixed-lineage leukemia treated with desensitization therapy prior to HLA–haploidentical transplantation with high donor-specific antibodies
International Journal of Hematology (2024)
-
A Single Centre Experience of Effective Desensitization Strategy for Children with High Anti-HLA Donor-Specific Antibodies Undergoing Haploidentical Hematopoietic Stem Cell Transplantation
Indian Journal of Hematology and Blood Transfusion (2024)
-
Effects of donor-specific antibodies on engraftment and long-term survival after allogeneic hematopoietic stem cell transplantation—A systematic review and meta-analysis
Bone Marrow Transplantation (2023)
-
The impact of HLA donor-specific antibodies on engraftment and the evolving desensitization strategies
Bone Marrow Transplantation (2022)
-
Successful desensitization of high level donor-specific anti-HLA antibody in patients with hematological diseases receiving haploidentical allografts
Annals of Hematology (2022)