Abstract
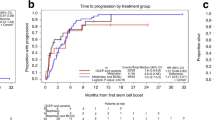
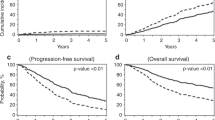
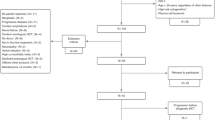
Modern combinations of therapies for multiple myeloma have led to improvement in survival outcomes with near 100% overall response rate and 25% complete response rates, particularly with autologous hematopoietic cell transplant (AHCT). Minimal residual disease (MRD) assessment with multiparameter flow cytometry is a valid prognostic biomarker for progression-free survival (PFS) and overall survival (OS). However, few data exist regarding whether MRD positivity or negativity will meaningfully influence treatment decisions. We evaluated 433 patients who received induction therapy, followed by AHCT. Participants had MRD assessment by multiparameter flow cytometry before and at days +100 and +365 following AHCT. They also received either lenalidomide, bortezomib, or no maintenance therapy following AHCT. Maintenance treatment with lenalidomide improved MRD negativity at day +365 compared to bortezomib (92.9% vs 41.6%, p = 0.01), or no maintenance therapy (92.9% vs 24.4%, p = 0.012). The median PFS for patients who were MRD negative at day + 365 was 42 vs 17.5 months (p < 0.001) and median OS was 80.6 vs 59 months (p = 0.02). Maintenance therapy following AHCT for multiple myeloma improves the depth of response as assessed by MRD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Kumar S, Rajkumar S, Dispenzieri A, Lacy M, Hayman S, Buadi F, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Bianchi G, Richardson P, Anderson K. Promising therapies in multiple myeloma. Blood. 2015;126:300–10.
Anderson K, Alsina M, Atanackovic D, Biermann J, Chandler J, Costello C, et al. NCCN guidelines insights, multiple myeloma, version 3.2016. J Natl Compr Cancer Netw. 2016;14:389–400.
Child J, Morgan G, Davies F, Owen R, Bell S, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
Fermand J, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–6.
Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroup Francophone du Myelome. J Clin Oncol. 2014;32:2712–8.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Chanan-Khan A, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612–24.
Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med. 2017;281:365–82.
Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: a meta-analysis. Bone Marrow Transplant. 2016;51:1565–8.
Munshi N, Avet-Loiseau H, Rawstron A, Owen R, Child J, Thakurta A, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3:28–35.
Sherrod A, Hari P, Mosse C, Walker R, Cornell R. Minimal residual disease testing after stem cell transplantation for multiple myeloma. Bone Marrow Transplant. 2016;51:2–12.
Paiva B, van Dongen J, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125:3059–68.
Anderson K. Should minimal residual disease negativity be the end-point of myeloma therapy? Blood Adv. 2017;1:517–21.
Paiva B, Gutierrez N, Rosinol L, Vidriales M, Montalban M, Martinez-Lopez J, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687–91.
Paiva B, Vidriales M, Cervero J, Mateo G, Perez J, Montalban M, et al. Multiparameter flow cytometry remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–23.
Rawstron A, Child J, de Tute R, Davies F, Gregory W, Bell S, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–7.
Hart A, Jagasia M, Kim A, Mosse C, Savani B, Kassim A. Minimal residual disease in myeloma: are we there yet? Biol Blood Marrow Transplant. 2012;18:1790–9.
Durie B, Harousseau J, Miguel J, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Kyle R, Rajkumar S. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9.
Kumar S, Paiva B, Anderson K, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Holstein S, Avet-Loiseau H, Hahn T, Ho C, Lohr J, Munshi N, et al. BMT CTN Myeloma Intergroup workshop on minimal residual disease and immune profiling: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. 2018;24:641–8.
Solovev M, Mendeleeva L, Pokrovskaya O, Gemdzhian E, Kuzmina L, Firsova M, et al. Maintenance therapy after autologous hematopoietic stem cell transplantation (auto-HSCT) in multiple myeloma patients with and without minimal residual disease (MRD). Blood. 2016;128:2260.
Sengsayadeth S, Malard F, Savani B, Garderet L, Mohty M. Posttransplant maintenance therapy in multiple myeloma: the changing landscape. Blood Cancer J. 2017;7:e545. https://doi.org/10.1038/bcj.2017.23.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91.
Huang J, Phillips S, Byrne M, Chinratanalab W, Engelhardt B, Goodman S, et al. Lenalidomide vs bortezomib maintenance choice post-autologous hematopoietic cell transplantation for multiple myeloma. Bone Marrow Transplant. 2018;53:701–7.
Sonneveld P. Should minimal residual disease negativity not be the end point of myeloma therapy. Blood Adv. 2017;1:522–5.
de Tute R, Rawstron A, Gregory W, Child J, Davies F, Bell S, et al. Minimal residual disease following autologous stem cell transplant in myeloma: impact on outcome is independent of induction regimen. Haematologica. 2016;101:e69–71.
McCarthy P, Holstein S, Petrucci M, Richardson P, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Jackson G, Davies F, Pawlyn C, Cairns D, Striha A, Collett C, et al. Lenalidomide maintenance vs observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;20:P57–73.
Fernandez R, Cedena M, Rios R, Jimenez J, Sanz A, Martin F, et al. Maintenance treatment with lenalidomide for multiple myeloma increases the proportion of MRD negative (Flow-/PET-CT-) patients. Blood. 2017;130:3098.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson K, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Sivaraj D, Green M, Li Z, Sung A, Sarantopoulos S, Kang Y, et al. Outcomes of maintenance therapy with bortezomib after autologous stem cell transplantation for patients with multiple myeloma. Biol Blood Marrow Transplant. 2017;23:262–8.
Mian I, Milton D, Shah N, Nieto Y, Popat U, Kebriaei P, et al. Prolonged survival with a longer duration of maintenance lenalidomide after autologous hematopoietic stem cell transplantation for multiple myeloma. Cancer. 2016;122:3831–7.
Mailankody S, Korde N, Lesokhin A, Lendvai N, Hassoun H, Stetler-Stevenson M, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015;12:286–95.
Landgren O, Owen R. Better therapy requires better response evaluation: paving the way for minimal residual disease testing for every myeloma patient. Cytom B Clin Cytom. 2016;90B:14–20.
Flanders A, Stetler-Stevenson M, Landgren O. Minimal residual disease testing in multiple myeloma by flow cytometry: major heterogeneity. Blood. 2013;122:1088–9.
Zhao X, Huang Q, Slovak M, Weiss L. Comparison of ancillary studies in the detection of residual disease in plasma cell myeloma in bone marrow. Am J Clin Pathol. 2006;125:895–904.
Roschewski M, Stetler-Stevenson M, Yuan C, Mailankody S, Korde N, Landgren O. Minimal residual disease: what are the minimum requirements? J Clin Oncol. 2014;32:475–6.
Martinez-Lopez J, Lahuerta J, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–9.
Dimopoulos M, Oriol A, Nahi H, San-Miguel J, Bahlis N, Usmani S, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Rossi G, Falcons A, Minervini M, De Cillis G, De Waure C, Sisti L. et al. Minimal residual disease and log-reduction of plasma cells are associated with superior response after double autologous stem cell transplant in younger patients with multiple myeloma. Cytom B Clin Cytom. 2019;96:195–200.
Acknowledgements
The authors would like to acknowledge all of the staff at Vanderbilt University Medical Center and Vanderbilt Ingram Cancer Center for their work in caring for patients with multiple myeloma. Statistical analysis performed by Ragisha Gopalakrishnan and Evonne McArthur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, D.A., Gopalakrishnan, R., Engelhardt, B.G. et al. Minimal residual disease negativity and lenalidomide maintenance therapy are associated with superior survival outcomes in multiple myeloma. Bone Marrow Transplant 55, 1137–1146 (2020). https://doi.org/10.1038/s41409-020-0791-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0791-y
This article is cited by
-
Cellular Immunotherapies for Multiple Myeloma: Current Status, Challenges, and Future Directions
Oncology and Therapy (2022)