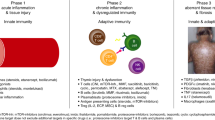
Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for patients with a hematologic disease. Although the clinical outcomes after allo-HCT have significantly improved during the last few decades, graft-versus-host disease (GVHD) is still a major cause of post-HCT morbidity and mortality. Systemic glucocorticoids (GC) remain an integral part of treatment in patients with GVHD including both acute and chronic GVHD. Although it is well-known that usage of systemic GC is associated with various side effects, the short- and long-term effects of GCs in the HCT setting are not well-characterized due to limited published data. In order to clarify this issue, we summarize the information on side effects associated with GCs, focusing specifically on the sequelae of these agents in the early post-HCT period. In instances where limited data are available, we included data from other fields such as autoimmune diseases, given the potential parallels between autoimmune conditions and GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bair SM, Brandstadter JD, Ayers EC, Stadtmauer EA. Hematopoietic stem cell transplantation for blood cancers in the era of precision medicine and immunotherapy. Cancer. 2020;126:1837–55.
Pavletic SZ, Pusic I. Challenges in conducting studies in chronic graft-versus-host disease. Clin Hematol Int. 2019;1:36–44.
Al-Homsi AS, Cole K, Cirrone F, Abdul-Hay M, Choi J, Tegla C. Current status and future directions in graft-versus-host disease prevention following allogeneic blood and marrow transplantation in adults. Clin Hematol Int. 2020;2:5–12.
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2012;18:1150–63.
Luis M, Freitas J, Costa F, Buttgereit F, Boers M, Jap DS, et al. An updated review of glucocorticoid-related adverse events in patients with rheumatoid arthritis. Expert Opin Drug Saf. 2019;18:581–90.
McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20:131–7.
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800–10.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–67.
Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133:1191–200.
Modi A, Rybicki L, Majhail NS, Mossad SB. Severity of acute gastrointestinal graft-vs-host disease is associated with incidence of bloodstream infection after adult allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2020;22:e13217.
Mori Y, Yoshimoto G, Nishida R, Sugio T, Miyawaki K, Shima T, et al. Gastrointestinal graft-versus-host disease is a risk factor for postengraftment bloodstream infection in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transpl. 2018;24:2302–9.
Fuji S, Kapp M, Einsele H. Challenges to preventing infectious complications, decreasing re-hospitalizations, and reducing cost burden in long-term survivors after allogeneic hematopoietic stem cell transplantation. Semin Hematol. 2012;49:10–4.
Miller HK, Braun TM, Stillwell T, Harris AC, Choi S, Connelly J, et al. Infectious risk after allogeneic hematopoietic cell transplantation complicated by acute graft-versus-host disease. Biol Blood Marrow Transpl. 2017;23:522–8.
Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–84.
Fuji S, Rovo A, Ohashi K, Griffith M, Einsele H, Kapp M, et al. How do I manage hyperglycemia/post-transplant diabetes mellitus after allogeneic HSCT. Bone Marrow Transpl. 2016;51:1041–9.
Fuji S, Loffler J, Savani BN, Einsele H, Kapp M. Hyperglycemia as a possible risk factor for mold infections-the potential preventative role of intensified glucose control in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2017;52:657–62.
Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–63.
Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–34.
Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754–64.
Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2006;4:621–30.
Demetrakopoulos GE, Tsokos GC, Levine AS. Recovery of splenic function after GVHD-associated functional asplenia. Am J Hematol. 1982;12:77–80.
Al-Eid MA, Tutschka PJ, Wagner HN Jr., Santos GW, Tsan MF. Functional asplenia in patients with chronic graft-versus-host disease: concise communication. J Nucl Med. 1983;24:1123–6.
Altamura M, Caradonna L, Amati L, Pellegrino NM, Urgesi G, Miniello S. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol Immunotoxicol. 2001;23:153–61.
Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45.
Engelhard D, Cordonnier C, Shaw PJ, Parkalli T, Guenther C, Martino R, et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol. 2002;117:444–50.
Kulkarni S, Powles R, Treleaven J, Riley U, Singhal S, Horton C, et al. Chronic graft versus host disease is associated with long-term risk for pneumococcal infections in recipients of bone marrow transplants. Blood. 2000;95:3683–6.
Youssef S, Rodriguez G, Rolston KV, Champlin RE, Raad II, Safdar A. Streptococcus pneumoniae infections in 47 hematopoietic stem cell transplantation recipients: clinical characteristics of infections and vaccine-breakthrough infections, 1989–2005. Medecine. 2007;86:69–77.
Sayer HG, Longton G, Bowden R, Pepe M, Storb R. Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood. 1994;84:1328–32.
Ruutu T, Volin L, Parkkali T, Juvonen E, Elonen E. Cyclosporine, methotrexate, and methylprednisolone compared with cyclosporine and methotrexate for the prevention of graft-versus-host disease in bone marrow transplantation from HLA-identical sibling donor: a prospective randomized study. Blood. 2000;96:2391–8.
Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–94.
Mielcarek M, Furlong T, Storer BE, Green ML, McDonald GB, Carpenter PA, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100:842–8.
Sanders LA, Rijkers GT, Kuis W, Tenbergen-Meekes AJ, de Graeff-Meeder BR, Hiemstra I, et al. Defective antipneumococcal polysaccharide antibody response in children with recurrent respiratory tract infections. J Allergy Clin Immunol. 1993;91:110–9.
Sanders LA, Rijkers GT, Tenbergen-Meekes AM, Voorhorst-Ogink MM, Zegers BJ. Immunoglobulin isotype-specific antibody responses to pneumococcal polysaccharide vaccine in patients with recurrent bacterial respiratory tract infections. Pediatr Res. 1995;37:812–9.
Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Kruger W, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95:1435–55.
Kruger WH, Bohlius J, Cornely OA, Einsele H, Hebart H, Massenkeil G, et al. Antimicrobial prophylaxis in allogeneic bone marrow transplantation. Guidelines of the infectious diseases working party (AGIHO) of the german society of haematology and oncology. Ann Oncol. 2005;16:1381–90.
Flowers MED, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–15.
Maertens JA, Girmenia C, Bruggemann RJ, Duarte RF, Kibbler CC, Ljungman P, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73:3221–30.
Wald A, Leisenring W, van Burik JA, Bowden RA. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–66.
Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–66.
Matsumura-Kimoto Y, Inamoto Y, Tajima K, Kawajiri A, Tanaka T, Hirakawa T, et al. Association of cumulative steroid dose with risk of infection after treatment for severe acute graft-versus-host disease. Biol Blood Marrow Transpl. 2016;22:1102–7.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47.
Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128.
Huang L, Morris A, Limper AH, Beck JM. An Official ATS Workshop Summary: recent advances and future directions in pneumocystis pneumonia (PCP). Proc Am Thorac Soc. 2006;3:655–64.
De Castro N, Neuville S, Sarfati C, Ribaud P, Derouin F, Gluckman E, et al. Occurrence of pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transpl. 2005;36:879–83.
Chen CS, Boeckh M, Seidel K, Clark JG, Kansu E, Madtes DK, et al. Incidence, risk factors, and mortality from pneumonia developing late after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2003;32:515–22.
Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116:5476–85.
Cope AV, Sabin C, Burroughs A, Rolles K, Griffiths PD, Emery VC. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor-recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J Infect Dis. 1997;176:1484–90.
Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100:3690–7.
Aubert G, Hassan-Walker AF, Madrigal JA, Emery VC, Morte C, Grace S, et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis. 2001;184:955–63.
Lilleri D, Fornara C, Chiesa A, Caldera D, Alessandrino EP, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica. 2008;93:248–56.
Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7.
Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, et al. Clinical features of late cytomegalovirus infection after hematopoietic stem cell transplantation. Int J Hematol. 2008;87:310–8.
Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97:867–74.
Bowen EF, Wilson P, Cope A, Sabin C, Griffiths P, Davey C, et al. Cytomegalovirus retinitis in AIDS patients: influence of cytomegaloviral load on response to ganciclovir, time to recurrence and survival. Aids. 1996;10:1515–20.
Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhauser M, Groth C, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370:1781–9.
Merchant SL, Gatwood KS, Satyanarayana G, Byrne MT, Kassim AA, Jagasia M, et al. Efficacy and pharmacoeconomic impact of letermovir for CMV prophylaxis in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2019;25:S280.
Gupta A, Gupta Y. Glucocorticoid-induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17:913–6.
Minetto MA, Lanfranco F, Motta G, Allasia S, Arvat E, D’Antona G. Steroid myopathy: some unresolved issues. J Endocrinol Invest. 2011;34:370–5.
Natsui K, Tanaka K, Suda M, Yasoda A, Sakuma Y, Ozasa A, et al. High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int. 2006;17:105–8.
Fardet L, Flahault A, Kettaneh A, Tiev KP, Genereau T, Toledano C, et al. Corticosteroid-induced clinical adverse events: frequency, risk factors and patient’s opinion. Br J Dermatol. 2007;157:142–8.
Nava S, Fracchia C, Callegari G, Ambrosino N, Barbarito N, Felicetti G. Weakness of respiratory and skeletal muscles after a short course of steroids in patients with acute lung rejection. Eur Respir J. 2002;20:497–9.
Qian T, Guo X, Levi AD, Vanni S, Shebert RT, Sipski ML. High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005;43:199–203.
Hopkinson NS, Man WD, Dayer MJ, Ross ET, Nickol AH, Hart N, et al. Acute effect of oral steroids on muscle function in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:137–42.
Takekiyo T, Dozono K, Mitsuishi T, Murayama Y, Maeda A, Nakano N, et al. Effect of exercise therapy on muscle mass and physical functioning in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2015;23:985–92.
Morishita S, Kaida K, Yamauchi S, Sota K, Ishii S, Ikegame K, et al. Relationship between corticosteroid dose and declines in physical function among allogeneic hematopoietic stem cell transplantation patients. Support Care Cancer. 2013;21:2161–9.
Braith RW, Welsch MA, Mills RM Jr., Keller JW, Pollock ML. Resistance exercise prevents glucocorticoid-induced myopathy in heart transplant recipients. Med Sci Sports Exerc. 1998;30:483–9.
Sosa M, Gomez de Tejada MJ. Glucocorticoid-induced osteoporosis. N Engl J Med. 2019;380:1378–9.
Al-Sari UA, Tobias J, Clark E. Health-related quality of life in older people with osteoporotic vertebral fractures: a systematic review and meta-analysis. Osteoporos Int. 2016;27:2891–900.
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology. 2000;39:1383–9.
Amiche MA, Abtahi S, Driessen JHM, Vestergaard P, de Vries F, Cadarette SM, et al. Impact of cumulative exposure to high-dose oral glucocorticoids on fracture risk in Denmark: a population-based case-control study. Arch Osteoporos. 2018;13:30.
De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 2007;56:208–14.
Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22:809–16.
Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2012;18:348–71.
Kendler DL, Body JJ, Brandi ML, Broady R, Cannata-Andia J, Cannata-Ortiz MJ, et al. Bone management in hematologic stem cell transplant recipients. Osteoporos Int. 2018;29:2597–610.
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018;6:445–54.
Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol. 2019;71:1174–84.
Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–55.
Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–39.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43.
Goodwin JE, Geller DS. Glucocorticoid-induced hypertension. Pediatr Nephrol. 2012;27:1059–66.
Mantero F, Boscaro M. Glucocorticoid-dependent hypertension. J Steroid Biochem Mol Biol. 1992;43:409–13.
Gale S, Wilson JC, Chia J, Trinh H, Tuckwell K, Collinson N, et al. Risk associated with cumulative oral glucocorticoid use in patients with giant cell arteritis in real-world databases from the USA and UK. Rheumatol Ther. 2018;5:327–40.
Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34:1519–27.
Gavriilaki E, Gkaliagkousi E, Sakellari I, Anyfanti P, Douma S, Anagnostopoulos A. Early prediction of cardiovascular risk after hematopoietic cell transplantation: are we there yet? Biol Blood Marrow Transpl. 2019;25:e310–6.
Leger KJ, Baker KS, Cushing-Haugen KL, Flowers MED, Leisenring WM, Martin PJ, et al. Lifestyle factors and subsequent ischemic heart disease risk after hematopoietic cell transplantation. Cancer. 2018;124:1507–15.
DeFilipp Z, Duarte RF, Snowden JA, Majhail NS, Greenfield DM, Miranda JL, et al. Metabolic syndrome and cardiovascular disease after hematopoietic cell transplantation: screening and preventive practice recommendations from the CIBMTR and EBMT. Biol Blood Marrow Transpl. 2016;22:1493–503.
Matheny L, Leon BGC, Savani B, Eplin D, Matthews J. Managing endocrine disorders in adults after hematopoietic stem. Cell Transplant Clin Hematol Int. 2019;1:180–8.
Pidala J, Hamadani M, Dawson P, Martens M, Alousi AM, Jagasia M, et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood. 2020;135:97–107.
Frairia C, Nicolosi M, Shapiro J, Kim J, Betts BC, Fernandez HF, et al. Sole upfront therapy with beclomethasone and budesonide for upper gastrointestinal acute graft-versus-host disease. Biol Blood Marrow Transpl. 2020;26:1303–11.
McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng GS, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. 2020;172:229–39.
Acknowledgements
This study was partly supported by Health, Labour and Welfare Sciences Research Grants (20FF1002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fuji, S., Byrne, M., Nagler, A. et al. How we can mitigate the side effects associated with systemic glucocorticoid after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 56, 1248–1256 (2021). https://doi.org/10.1038/s41409-020-01205-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01205-6
This article is cited by
-
Steroid-free first line treatment of moderate and severe chronic GVHD: a survey from the Transplant Complications Working Party of the EBMT
Bone Marrow Transplantation (2023)
-
Graft-Versus-Host Disease: an Update on Functional Implications and Rehabilitation Interventions
Current Oncology Reports (2023)
-
Impact of Resistance Exercise and Nutritional Endorsement on physical performance in patients with GvHD (IRENE-G study) – design and rational of a randomized controlled trial
BMC Cancer (2022)