Abstract
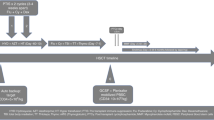
High-dose cyclophosphamide given post-transplant (PTCy) successfully enables tolerance induction in HLA-mismatched related blood or marrow transplantation (haploBMT) manifested by low rates of graft failure, severe acute graft-versus-host disease (GVHD), and chronic GVHD. When proceeded by nonmyeloablative conditioning, PTCy has also been associated with a low incidence of nonrelapse mortality. The safety of this platform has garnered interest in expanding its use to non-malignant indications for allogeneic blood or marrow transplantation (alloBMT). After success in a preliminary Phase I/II trial, use of a PTCy-based haploBMT platform is now being explored in a large Blood and Marrow Transplant Clinical Trials Network (BMT CTN) study for sickle cell disease. These emerging data in patients with hemoglobinopathies provided the rationale for exploring the use of PTCy in combined solid organ and BM transplantation as a means of tolerance induction through donor hematopoietic chimerism with a goal to obviate the need for a lifetime of immunosuppression. Several case reports, series, and small clinical trials have now been published of combined solid organ and alloBMT in patients with hematologic malignancies who had organ failure that would have been preclusive of alloBMT in the absence of solid organ transplantation. Here we will review the pre-clinical and clinical studies supporting the use of PTCy for chimerism-based tolerance induction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Strober S. Use of hematopoietic cell transplants to achieve tolerance in patients with solid organ transplants. Blood. 2016;127:1539–43.
Mahr B, Granofszky N, Muckenhuber M, Wekerle T. Transplantation tolerance through hematopoietic chimerism: progress and challenges for clinical translation. Front Immunol. 2017;8:1762.
Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23:165–73.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8.
McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolaños-Meade J, Rosner GL et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–31.
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64.
Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–8.
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93.
Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–38.
Owens AH Jr., Santos GW. The effect of cytotoxic drugs on graft-versus-host disease in mice. Transplantation. 1971;11:378–82.
Yolcu ES, Shirwan H, Askenasy N. Mechanisms of Tolerance induction by hematopoietic chimerism: the immune perspective. Stem Cells Transl Med. 2017;6:700–12.
Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125:2855–64.
Cieri N, Oliveira G, Greco R, Forcato M, Taccioli C, Cianciotti B et al. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood. 2015;125:2865–74.
Ross D, Jones M, Komanduri K, Levy RB. Antigen and lymphopenia-driven donor T cells are differentially diminished by post-transplantation administration of cyclophosphamide after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1430–8.
Kanakry CG, Ganguly S, Zahurak M, Bolaños-Meade J, Thoburn C, Perkins B et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157.
Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazer BR, Levy RB et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124:2131–41.
McCurdy SR, Kanakry CG, Tsai HL, Kasamon YL, Showel MM, Bolaños-Meade J et al. Grade II acute graft-versus-host disease and higher nucleated cell graft dose improve progression-free survival after HLA-haploidentical transplant with post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2018;24:343–52.
Kanakry CG, Coffey DG, Towlerton AM, Vulic A, Storer BE, Chou J et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight. 2016;1:e86252.
Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–9.
McCurdy SR, Kasamon YL, Kanakry CG, Bolaños-Meade J, Tsai HL, Showel MM et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica. 2017;102:391–400.
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40.
Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.
Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Kasamon YL, Fuchs EJ, Zahurak M, Rosner GL, Symons HJ, Gladstone DE et al. Shortened-duration tacrolimus after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2018;24:1022–8.
Kanakry CG, Bolaños-Meade J, Kasamon YL, Zahurak M, Durakovic N, Furlong T et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood. 2017;129:1389–93.
Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolaños-Meade J et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood. Adv 2017;1:288–92.
Pasquet L, Douet JY, Sparwasser T, Romagnoli P, van Meerwijk JP. Long-term prevention of chronic allograft rejection by regulatory T-cell immunotherapy involves host Foxp3-expressing T cells. Blood. 2013;121:4303–10.
Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–45.
Dodd OJ, Ganguly S, Vulic A, Panoskaltsis-Mortari A, McDyer JF, Luznik L. Induction of major histocompatibility complex-mismatched mouse lung allograft acceptance with combined donor bone marrow: lung transplant using a 12-hour nonmyeloablative conditioning regimen. Transplantation. 2016;100:e140–e146.
Kasamon YL, Bolaños-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA et al. Outcomes of nonmyeloablative hla-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol 2015;33:3152–61.
Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–6.
Brodsky RA, Luznik L, Bolaños-Meade J, Leffell MS, Jones RJ, Fuchs EJ. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42:523–7.
Bolaños-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–91.
Chen YB, Kawai T, Spitzer TR. Combined bone marrow and kidney transplantation for the induction of specific tolerance. Adv Hematol. 2016;2016:6471901.
Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra128.
Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott M et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95:169–76.
Leventhal JR, Ildstad ST. Tolerance induction in HLA disparate living donor kidney transplantation by facilitating cell-enriched donor stem cell Infusion: the importance of durable chimerism. Hum Immunol. 2018;79:272–6.
Funding
Publication of this supplement was sponsored by Gilead Sciences Europe Ltd, Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SRM received grant support from American Cancer Society Institutional Research Grant (ACS IRG) and served as PI for 129784-IRG-16-188-38-IRG. LL has received consulting fees from Pharmacyclics and Abbivie, lecture fees from Merck, grant support from Gennetech, and receives patent royalties on alloMILs (WindMill Therapheutics).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McCurdy, S.R., Luznik, L. Post-transplantation cyclophosphamide for chimerism-based tolerance. Bone Marrow Transplant 54 (Suppl 2), 769–774 (2019). https://doi.org/10.1038/s41409-019-0615-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0615-0
This article is cited by
-
Mafosfamide, a cyclophosphamide analog, causes a proinflammatory response and increased permeability on endothelial cells in vitro
Bone Marrow Transplantation (2023)
-
Long-term survival with mixed chimerism in patients with AML and MDS transplanted after conditioning with targeted busulfan, fludarabine, and thymoglobulin
Bone Marrow Transplantation (2022)