Abstract
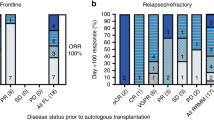
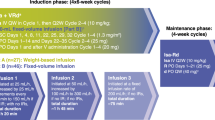
A regimen of escalating doses of thalidomide, in combination with bortezomib and high-dose melphalan (mel/vel/thal), was evaluated as a conditioning regimen for autologous stem cell transplantation (ASCT) in multiple myeloma (MM) patients with a prior transplant who had relapsed or achieved less than a complete remission following a prior ASCT. Thalidomide was dose escalated starting from 600 mg to 1000 mg on days −5 to −1 in a 3 × 3 design, bortezomib was administered at 1.6 mg/m2 intravenously on days −4 and −1 and melphalan 200 mg/m2 was administered on day −2. No dose-limiting toxicity was seen in the phase I portion of the trial. An additional 20 patients were enrolled at the maximum tolerated dose of thalidomide of 1000 mg daily. The overall response rate was 69% with 38% complete remission. Median PFS and OS were 9.3 and 65.4 months, respectively, with a median follow-up of 17.8 months. The most common grade 3–4 adverse events (AEs) were neutropenic fever (58.6%), mucositis (6.9%), and diarrhea (6.9%). Serious AEs included somnolence (13.8%) and tumor lysis syndrome (3.4%). The addition of high-dose thalidomide to bortezomib and melphalan as conditioning for salvage ASCT was well tolerated and was an effective conditioning regimen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Myeloma: Surveillance Epidemiology and End Results Program: SEER Stats Facts Sheet—Myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–8.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. New Engl J Med. 1996;335:91–7.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. New Engl J Med. 2003;348:1875–83.
McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–33.
Blade J, Rosinol L, Sureda A, Ribera JM, Diaz-Mediavilla J, Garcia-Larana J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–9.
Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–92.
Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–55.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. New Engl J Med. 2012;366:1770–81.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. New Engl J Med. 2012;366:1782–91.
Shah N, Ahmed F, Bashir Q, Qureshi S, Dinh Y, Rondon G, et al. Durable remission with salvage second autotransplants in patients with multiple myeloma. Cancer. 2012;118:3549–55.
Jimenez-Zepeda VH, Mikhael J, Winter A, Franke N, Masih-Khan E, Trudel S, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression-free and overall survival. Biol Blood Marrow Transplant. 2012;18:773–9.
Olin RL, Vogl DT, Porter DL, Luger SM, Schuster SJ, Tsai DE, et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43:417–22.
Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI, et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. 2013;19:760–6.
Desikan KR, Tricot G, Dhodapkar M, Fassas A, Siegel D, Vesole DH, et al. Melphalan plus total body irradiation (MEL-TBI) or cyclophosphamide (MEL-CY) as a conditioning regimen with second autotransplant in responding patients with myeloma is inferior compared to historical controls receiving tandem transplants with melphalan alone. Bone Marrow Transplant. 2000;25:483–7.
Biran N, Rowley SD, Vesole DH, Zhang S, Donato ML, Richter J, et al. A Phase I/II study of escalating doses of bortezomib in conjunction with high-dose melphalan as a conditioning regimen for salvage autologous peripheral blood stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2016;22:2165–71.
Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–14.
Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32:634–40.
Nadiminti K, Singh Abbi KK, Mott SL, Dozeman L, Tricot A, Schultz A, et al. VTD-melphalan is well tolerated and results in very high rates of stringent CR and MRD-negative status in multiple myeloma. OncoTargets Ther. 2017;10:217–26.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
CTEP. Common Terminology Criteria for Adverse Events version 3.0. 3.0: Common terminology for adverse events utilized for adverse events reporting. Accessed 2 March 2016. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.
Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7.
Sonneveld P, Salwender HJ, Van Der Holt B, el Jarari L, Bertsch U, W. Blau I, et al. Bortezomib induction and maintenance in patients with newly diagnosed multiple myeloma: long-term follow-up of the HOVON-65/GMMG-HD4 Trial. Orlando FL: American Society of Hematology; 2015.
Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. New Engl J Med. 2003;349:2495–502.
Cavo MGF, Patriarca F, Zamagni E, Montefusco V, Dozza L, Galli M, et al. Double autologous stem cell transplantation significantly prolongs progression-free survival and overall survival in comparison with single augotransplantation in newly diagnosed multiple myeloma: an analysis of phase 3 EMN02/H095 study. Abstract 401. Atlanta, GA: American Society of Hematology; 2017.
Fenk R, Liese V, Neubauer F, Bruns I, Kondakci M, Balleisen S, et al. Predictive factors for successful salvage high-dose therapy in patients with multiple myeloma relapsing after autologous blood stem cell transplantation. Leuk Lymphoma. 2011;52:1455–62.
Alvares CL, Davies FE, Horton C, Patel G, Powles R, Morgan GJ. The role of second autografts in the management of myeloma at first relapse. Haematologica. 2006;91:141–2.
Sellner L, Boumendil A, Finel H, Choquet S, de Rosa G, Falzetti F, et al. Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: a retrospective study from the EBMT. Bone Marrow Transplant. 2016;51:212–8.
Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:874–85.
Singh Abbi KK, Zheng J, Devlin SM, Giralt S, Landau H. Second autologous stem cell transplant: an effective therapy for relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015;21:468–72.
Giralt S, Garderet L, Durie B, Cook G, Gahrton G, Bruno B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant. 2015;21:2039–51.
Bianchi G, Ghobrial IM. Biological and clinical implications of clonal heterogeneity and clonal evolution in multiple myeloma. Curr Cancer Ther Rev. 2014;10:70–9.
Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo P, Lawrence MS, Auclair D, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25:91–101.
Costa LJ, Landau HJ, Chhabra S, Hari P, Innis-Shelton R, Godby KN, et al. Phase 1/2 trial of carfilzomib plus high-dose melphalan preparative regimen for salvage autologous hematopoietic cell transplantation followed by maintenance carfilzomib in patients with relapsed/refractory multiple myeloma. Biol Blood Marrow Transplant. 2018;24:1379–85.
Rodriguez TE, Hari P, Stiff PJ, Smith SE, Sterrenberg D, Vesole DH. Busulfan, melphalan, and bortezomib versus high-dose melphalan as a conditioning regimen for autologous hematopoietic stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2016;22:1391–6.
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood. 2010;115:32–7.
Roussel MHB, Lauwers-Cances V, Macro M, Leleu X, Caillot D, Rigaudeau S, et al. Bortezomib and high-dose melphalan vs. high-dose melphalan as conditioning regimen before autologous stem cell transplantation in de novo multiple myeloma patients: a phase 3 study of the Intergroupe Francophone Du Myelome (IFM 2014-02). Atlanta, GA: American Society of Hematology; 2017.
Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569–74.
Fouquet G, Hebraud B, Garciaz S, Stoppa AM, Roussel M, Caillot D, et al. Partial response at completion of bortezomib-thalidomide-dexamethasone (VTd) induction regimen upfront in multiple myeloma does not preclude response to VTd in consolidation. J Cancer. 2014;5:248–52.
Leleu X, Fouquet G, Hebraud B, Roussel M, Caillot D, Chretien ML, et al. Consolidation with VTd significantly improves the complete remission rate and time to progression following VTd induction and single autologous stem cell transplantation in multiple myeloma. Leukemia. 2013;27:2242–4.
Garderet L, Iacobelli S, Moreau P, Dib M, Lafon I, Niederwieser D, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–82.
Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–96.
Mercurio A, Adriani G, Catalano A, Carocci A, Rao L, Lentini G, et al. A mini-review on thalidomide: chemistry, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr Med Chem. 2017;24:2736–44.
Holstein SA, McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. 2017;77:505–20.
Palumbo A, Avonto I, Bruno B, Falcone A, Scalzulli PR, Ambrosini MT, et al. Intermediate-dose melphalan (100 mg/m2)/bortezomib/thalidomide/dexamethasone and stem cell support in patients with refractory or relapsed myeloma. Clin Lymphoma Myeloma. 2006;6:475–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
NB—Speaker’s bureau for Celgene, Takeda, Amgen, and Janssen; Advisory Board for Takeda, Celgene, and Amgen; Research funding from Merck and Bristol Meyers Squibb; DHV—Speaker’s bureau for Takeda, Amgen, Celgene, and Janssen; JR—Speaker’s bureau for Celgene, Takeda, Janssen, Amgen, Sanofi, and BMS; DSS—Speaker’s bureau for Celgene, Takeda, and Amgen; Advisory Board and Consulting for Takeda, Celgene, Amgen, and Janssen. The other authors declare that they have no conflict of interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biran, N., Rowley, S.D., Vesole, D.H. et al. A phase I/II study of escalating doses of thalidomide in conjunction with bortezomib and high-dose melphalan as a conditioning regimen for autologous stem cell transplantation in patients with multiple myeloma. Bone Marrow Transplant 54, 1881–1891 (2019). https://doi.org/10.1038/s41409-019-0534-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0534-0