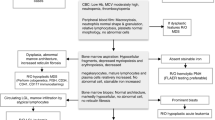
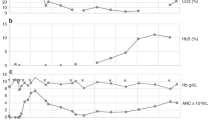
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation (HSCT). This study evaluated clinical and morphological practices of TA-TMA diagnosis in EBMT centers. Two questionnaires, one for transplant physician and one for morphologist, and also a set of electronic blood slides from 10 patients with TA-TMA and 10 control patients with various erythrocyte abnormalities, were implemented for evaluation. Seventeen EBMT centers participated in the study. Regarding criteria used for TA-TMA diagnosis, centers reported as follows: 41% of centers used the International Working Group (IWG) criteria, 41% used “overall TA-TMA” criteria and 18% used physician’s decision. The threshold of schistocytes to establish TA-TMA diagnosis in the participating centers was significantly associated with morphological results of test cases evaluations (p = 0.002). The mean number of schistocytes reported from blood slide analyses were 4.3 ± 4.5% for TA-TMA cases (range 0–19.6%, coefficient of variation (CV) 0.7) and 1.3 ± 1.6% for control cases (range 0–8.3%, CV 0.8). Half of the centers reported schistocyte levels below 4% for 7/10 TA-TMA cases. The intracenter variability was low, indicating differences in the institutional practices of morphological evaluation. In conclusion, the survey identified the need for the standardization of TA-TMA morphological diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol. 2009;4:345–53.
Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–53.
Gloude NJ, Khandelwal P, Luebbering N, Lounder DT, Jodele S, Alder MN, et al. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood. 2017;130:1259–66.
Mulay S, Kreuter JD, Bryant SC, Elliott MA, Hogan WJ, Winters JL, et al. Outcomes of plasma exchange in patients with transplant-associated thrombotic microangiopathy based on time of presentation since transplant. J Clin Apher. 2015 ;30:147–53.
de Fontbrune FS, Galambrun C, Sirvent A, Huynh A, Faguer S, Nguyen S, et al. Use of eculizumab in patients with allogeneic stem cell transplant-associated thrombotic microangiopathy: a study from the SFGM-TC. Transplantation. 2015;99:1953–9.
Kraft S, Bollinger N, Bodenmann B, Heim D, Bucher C, Lengerke C et al. High mortality in hematopoietic stem cell transplant-associated thrombotic microangiopathy with and without concomitant acute graft-versus-host disease. Bone Marrow Transplant. 2018. https://doi.org/10.1038/s41409-018-0293-3. [Epub ahead of print].
Vasu S, Wu H, Satoskar A, Puto M, Roddy J, Blum W, et al. Eculizumab therapy in adults with allogeneic hematopoietic cell transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. 2016;51:1241–4.
Fujiwara H, Maeda Y, Sando Y, Nakamura M, Tani K, Ishikawa T, et al. Treatment of thrombotic microangiopathy after hematopoietic stem cell transplantation with recombinant human soluble thrombomodulin. Transfusion. 2016;56:886–92.
Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G, et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci. 2016;54:181–90.
Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–5.
Ruutu T, Barosi G, Benjamin RJ, Clark RE, George JN, Gratwohl A, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100.
Hahn T, Alam AR, Lawrence D, Ford L, Baer MR, Bambach B, et al. Thrombotic microangiopathy after allogeneic blood and marrow transplantation is associated with dose-intensive myeloablative conditioning regimens, unrelated donor, and methylprednisolone T-cell depletion. Transplantation. 2004;78:1515–22.
Martinez MT, Bucher Ch, Stussi G, Heim D, Buser A, Tsakiris DA, et al. Transplant-associated microangiopathy (TA-TMA) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;36:993–1000.
Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:551–7.
Hale GA, Bowman LC, Rochester RJ, Benaim E, Heslop HE, Krance RA, et al. Hemolytic uremic syndrome after bone marrow transplantation: clinical characteristics and outcome in children. Biol Blood Marrow Transplant. 2005;11:912–20.
Willems E, Baron F, Seidel L, Frere P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45:689–93.
Glezerman IG, Jhaveri KD, Watson TH, Edwards AM, Papadopoulos EB, Young JW, et al. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:976–84.
Platzbecker U, von Bonin M, Goekkurt E, Radke J, Binder M, Kiani A, et al. Graft-versus-host disease prophylaxis with everolimus and tacrolimus is associated with a high incidence of sinusoidal obstruction syndrome and microangiopathy: results of the EVTAC trial. Biol Blood Marrow Transplant. 2009;15:101–8.
Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–26.
Labrador J, Lopez-Anglada L, Perez-Lopez E, Lozano FS, Lopez-Corral L, Sanchez-Guijo FM, et al. Analysis of incidence, risk factors and clinical outcome of thromboembolic and bleeding events in 431 allogeneic hematopoietic stem cell transplantation recipients. Haematologica. 2013;98:437–43.
Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–62.
G. Zini, G. d’onofrio, C. Briggs, W. Erber, J. M. Jou, S. H. Lee, S. McFadden, J. L. Vives-Corrons, N. Yutaka, J. F. Lesesve. ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. International Journal of Laboratory Hematology. 2012;34:107-116
Lesesve JF, Alla F, Dugue F, Salignac S, Clement L, Lecompte T, et al. Evaluation of schistocyte monitoring after haematopoietic stem cell transplantation. Int J Lab Hematol. 2011;33:343–56.
Lesesve JF, Speyer E, Perol JP. Fragmented red cells reference range for the Sysmex XN®-series of automated blood cell counters. Int J Lab Hematol. 2015;37:583–7.
Acknowledgements
Special thanks to Valentina Kravcova, Tatyana Schegoleva and Larena Darmilova for the collection of blood slides for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ISM had received travel grants from MSD, Novartis, Pfizer, Celgene, Takeda, BMS, consulting fees from Novartis and Celgene, lecturer fees from Novartis. OP has received honoraria and travel support from Astellas, Gilead, Jazz, MSD, Neovii Biotech and Pfizer. He has received research support from Bio Rad, Gilead, Jazz, Neovii Biotech, Pierre Fabre, Sanofi and Takeda. He is member of the advisory board to Alexion, Jazz, Gilead, MSD and Omeros. GWB is the member of the advisory board to Omeros. The other authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moiseev, I.S., Tsvetkova, T., Aljurf, M. et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant 54, 1022–1028 (2019). https://doi.org/10.1038/s41409-018-0374-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0374-3
This article is cited by
-
Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis
Bone Marrow Transplantation (2021)
-
Clinical usefulness of diagnostic criteria for transplant-associated thrombotic microangiopathy
International Journal of Hematology (2020)