Abstract
Introduction
The application of different cavity disinfectants is an essential step that eliminates bacteria after cavity preparation. However, some of these materials may affect restoration sealing ability.
Aim
This study aimed to assess the degree of microleakage at the tooth restoration interface after using different nano prepared cavity disinfectants versus Diode Laser.
Materials and methods
Three disinfectants were prepared on the nanoscale; Propolis, Liquorice and Chlorhexidine. A total of 40 extracted premolars with standard class V cavities were prepared on the facial surface. Teeth were divided into five groups according to the applied cavity disinfection protocol; no treatment, Chloehexidine, Propolis, Liquorice, and Diode Laser. After application of composite resin restoration, all teeth were subjected to thermocycling, afterwords the degree of microleakage was measured in micrometers. Data were analyzed by one-way analysis of variance (ANOVA) test, followed by Bonferroni’s and Tukey’s post hoc test.
Results
The highest mean microlekage value was recorded in no treatment group, followed by Liquorice, Propolis. While Diode Laser group showed the lowest degree of microleakage.
Conclusion
Diode Laser cavity disinfectant has no negative effect on the restoration sealing ability. Nano prepared Propolis showed comparative results to nanoChloehexidine as both had low degree of microleakage.
Similar content being viewed by others
Introduction
Dental caries has a high prevalence worldwide, it can be defined as a multifactorial pathology arising from the interaction between dental structure and microbial biofilm, due to the imbalance between remineralization and demineralization process, with the last one prevailing [1]. During cavity preparation, incomplete elimination of the cariogenic bacteria may lead to their survival for a long time causing secondary caries and restoration failure as well [2, 3].
Cavity disinfection has been suggested to eliminate or even reduce the microbial load just before the restorative procedure [4, 5]. Chlorhexidine (CHX) is considered the golden standard for cavity disinfection due to the antimicrobial effect and its ability of binding to bacterial,s amino acids [6]. Lacerda-Santos et al. [7] and Satpute et al. [8] have reported that up to 10% concentrations are acceptable to be in contact with living tissues.
Recently, there has been a growing need to replace chemical products with natural remedies as a part of dental therapeutics [9]. Propolis is a well-known natural substance extracted from bee hives with antibacterial property and others, accordingly, it is widely used in dentistry as a root canal disinfectant, mouth rinse, cavity disinfectant and others [10, 11]. Also, Liquorice has been used for different medicinal purposes, while the flavonoid component of its extract has strong antibacterial effect [11].
On the other hand, Lusche et al. [12] proved that the action of liquid antiseptics is restricted as their penetration inside the dentine is limited. Thus, it is important to evaluate depth-effective disinfection alternatives that show minimal side effects without forming bacterial resistance. Pharmaceutical scientists use various methods to enhance the dissolution and bioavailability of poorly soluble drugs such as; micronization, self-emulsification, salt formation, solid lipid nanoparticles nanocrystallization, and others [13, 14]. Awan et al. [14] stated that nanocrystallization enhances solubility and loading of the drug as well as increases its bioavailability.
Moreover, Lasers have become increasingly popular in dentistry because of their wide application. It can penetrate and kill bacteria in addition to occlusion of the dentinal tubules and thereby closing all the avenues of bacterial reinfection [15, 16]. Also, the antimicrobial effect of Diode laser significantly reduced the number of viable bacteria in the biofilm [17]. However, there are scarce data in the literature concerning the effect of Diode laser cavity disinfectant on restoration sealing ability.
Cavity disinfectants should not only have a strong antimicrobial effect but also must not compromise adhesion to the dental tissues, otherwise, it may lead to microleakage at the tooth-restoration interface [18]. Once the sealing ability is disrupted and marginal leakage occurred this consequently ends up with restoration failure [19, 20].
It was found that disinfectants may affect the adhesion of composite to dentine either positively or negatively, consequently affecting the clinical success of the restoration. Some materials have been claimed to increase the adhesion while others had a negative impact on the adhesion [5]. Moreover, there is scarce information in the literature concerning the impact of using different cavity disinfections on the degree of tooth –restoration microleakage [10].
Hence it was of interest to prepare different nanogrels; Chlorhexidine, Propolis, and Liquirose then study and compare their effect on composite microleakage versus Diode laser. The null hypothesis assumed that all of the applied cavity disinfectant protocols could not affect the sealing ability at the tooth-restoration interface negatively.
Materials and methods
I-Materials
See Table 1 for material information used in the study.
II- Methods
Ethical approval
This in vitro study was approved by the Institutional Review Board at Misr University for Science and Technology with approval number: 2022/0066.
Sample size calculation
Based on Bin‑Shuwaish et al. [21] using the G power statistical power. Analysis program (version 3.1.9.4) for sample size determination [22], the total sample size was 40 (8 in each group) which was sufficient to detect a large effect size (f) = 0.61, with an actual power (1-β error) of 0.8 (80%) and a significance level (α error) 0.05 (5%).
Grouping of samples
Group 1
No disinfection was applied (negative control) [6].
Group 2
In total, 2% Chlorhexidine nanogel (positive control): The cavity was dried with an air syringe and 1 ml of the prepared gel was applied for 60 s then it was washed with saline for 30 s [2].
Group 3
Propolis nanogel 10% was applied for 120 s then it was washed with saline for 30 s [2, 11]
Group 4
Liquorice nanogel 10% was applied for 120 s then it gel was washed with saline for 30 s [11].
Group 5
Diode laser (Doctor Smile, Wiser, LAMBDA SpA, Italy)
Diode Laser criteria: 980 nm waves, and 1 Watt output power was applied in a continuous manner for 60 s divided into 2 cycles. Each of these cycles was 30 s using a 200 µm diameter fiber optic tip. The tip was perpendicularly directed away from the prepared cavity by 1 mm distance [2, 10].
Preparation of samples
A total of 40 intact human premolar teeth recently extracted for orthodontic reasons were collected. All teeth were cleaned and examined under a stereomicroscope to exclude fractured tooth then they were stored until the beginning of the experiment. Standardized class V cavities (3 mm mesiodistally ×2 mm occlusogingivally ×1.5 mm buccolingually) were prepared on the facial surface of each tooth using diamond bur (HoricoDiament, Germany), 1 mm occlusal to the cementoenamel junction to assure that cavosurface line angles were prepared within enamel. A new bur was used for every five teeth with a copious amount of water coolant. Digital caliber was used to standardized the cavity dimensions [6].
All teeth were randomly divided into five experimental groups (n = 8) according to the cavity disinfection protocol.
Herbal extract preparation
Propolis
Propolis extract was purchased from www.thehealthshop.com (Brazilian green bee Propolis, Uniflora, FDA Reg.#10760930494, Brazil).
Liquorice
Liquorice collection
Liquorice was purchased from a commercial herbal company (Al Fahd Apothecary, Egypt) and identified at the Pharmacognosy Department, Misr University for Science and Technology after permission number PG7. A voucher specimen of Liquorice under number 16.9.2020 was deposited in Pharmacognosy Laboratory, Faculty of Pharmacy, Cairo.
Liquorice extract preparation
In total, 200 gm of Liquorice leaves were chopped into pieces and inserted into an electric oven at 45 ± 5 °C for 24 h until complete dryness. Then it was ground using an electric miller to produce a fine powder. Each 150 gm powder was immersed in a flask containing 96% ethanol (300 mL) for 72 h at room temperature. The solution was filtered and evaporated using a rotary flask evaporator to obtain the well-concentrated extract. Finally, the extract was stored in the refrigerator at 4 °C until needed.
Nanogel preparation
Preparation of CHX, Propolis, and Liquorice nanocrystals(NCs) at the Pharmacognosy Department, Misr University for Science and Technology:
Both CHX (C22H30CL2N10, Nerol, Egypt) and Propolis nanocrystals NCs were separately prepared through antisolvent precipitation method followed by high-shear homogenization to obtain NCs of the required size [13, 14].
To prepare the solvent phase, 2% CHX, and 10% of each of the Propolis and Liquorice extracts were first dissolved in a suitable quantity of methanol separately. The prepared solvent phase was added dropwise into the antisolvent phase with a ratio of 1:10 solvent to antisolvent containing pre-screened surfactant which is the lipid phase containing Compritol 888 ATO, Span60 solid lipid nanoparticles (4% of total concentration) with a stirring speed of 1200 rpm at room temperature [14].
Each of the prepared formulas was subjected to rotary evaporation for 20 min at 100 rpm and 70 ◦C to evaporate the organic phase. Then they were subjected to a homogenizer at a speed of 7000 rpm for 20 min. The developed formulations were further freeze-dried to obtain CHX, Propolis, and Liquorice Nanocrystals dried powder [23].
Each formula was assessed for entrapment efficiency percentage (EE %), Particle size (PS), Zeta potential (ZP), and Polydispersity index (PDI) (Malvern Instruments, Ltd., UK). The prepared solid lipid nanoparticles (SLNs) were incorporated into the gel using 1% noveon. The prepared nanoparticles showed particle sizes ranging from 80 to 100 nm, all with a negative charge and PDI below 0.5. The EE% ranged from 85 to 90% using Cooling Centrifuge (Sigma 3 K 30, Germany).
Restoration
After cavity disinfection protocols, all cavities were etched using HV Etch (Bisco, USA) followed by bond application; Gluma Bond Universal (Heraeus Kulzer, Germany) according to the manufacturer’s instructions [24]. Then all cavities were restored with Charisma composite (Kulzer, Germany) and light cured according to the manufacturer’s instructions.
Thermocycling process
All teeth were immersed in distilled water at 37 °C for 24 h. Then they were incubated for 78 s (30 s at 55 °C, 10 s stop, 30 s at 5 °C, and 8 s to return the cycle to the starting point). A total of 500 thermal cycles were conducted (TC-300 Vafai Factory Thermocycler) [25].
Microleakage test
Samples preparation for dye penetration
After thermocycling, all teeth were dried and their apices were blocked with sticky wax to prevent dye penetration. Afterward, they were coated with two layers of nail varnish within approximately 2 mm of the tooth-restoration interface except for 1 mm gingivally in order to prevent dye penetration except at tooth-restoration interface [25]. Then all teeth were immersed in 2% methylene blue dye (Sparks, USA) for 24 h at room temperature. After dye penetration, teeth were rinsed and dried then sectioned longitudinally in buccolingual direction using a microtome (MTI Corporation, Richmond CA) [26].
Microleakage assessment
Assessment of linear dye penetration at tooth- restoration interface both occlusal and gingival was done using the stereomicroscope at 100X magnification (MA 100 Nikon stereomicroscope Japan with Omnimet image analysis software) to study the degree of microleakage in micrometers. The depth of dye penetration was measured by Omnimet image analysis software [27, 28].
Statistical analysis
Data management and statistical analysis were performed using the Statistical Package for Social Sciences (SPSS) version 18. Data were explored for normality by checking the data distribution and using Kolmogorov-Smirnov and Shapiro-Wilk tests which revealed that most data were normally distributed (Table 2). Numerical data were summarized using mean, standard deviation, and confidence intervals. Comparisons between groups with respect to normally distributed numeric variables were compared by one-way analysis of variance (ANOVA) test, followed by Bonferroni’s and Tukey’s post hoc test. All p values are two-sided. P values ≤ 0.05 were considered significant.
Results
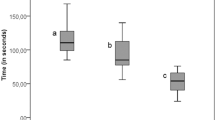
Microleakage results were summarized in (Tables 2, 3) and Fig. (1). Figures (2, 3, 4, 5, 6) represent digital images of dye penetration along the tooth- restoration interface.
I-At occlusal margin of the cavity:
The highest mean value was recorded in no treatment group, followed by Liquorice, Propolis and CHX groups respectively, with the lowest value recorded in Diode group. The difference between all groups was statistically significant (p = 0.00). Post hoc test revealed no significant difference between the no treatment, Liquorice, and Propolis groups. CHX group was not significantly different from Propolis nor from Diode groups.
II-At gingival margin of the cavity:
The highest mean value was recorded in Liquorice group, followed by no treatment, Propolis and Diode groups respectively, with the lowest value recorded in CHX group. The difference between all groups was statistically significant (p = 0.00). Post hoc test revealed no significant difference between the no treatment, Liquorice, and Propolis groups. Diode group was not significantly different from Propolis nor from CHX groups.
III-Average of microleakage both occlusally and gingivally:
The highest mean value was recorded in no treatment group, followed by Liquorice, Propolis and CHX groups respectively, with the lowest value recorded in Diode group. The difference between all groups was statistically significant (p = 0.00). Post hoc test revealed no significant difference between the no treatment group and Liquorice group. Diode group was not significantly different from CHX group.
Discussion
After cavity preparation cariogenic microorganisms can survive in the smear layer and dentin tubules, so complete eradication of the infected tissues should be done to guarantee the durability of the final restoration. Otherwise, secondary caries can occur that leads to restoration failure [29]. Several researchers have recommended using different cavity disinfection such as different chemical agents, laser, ozone, and others [19, 29,30,31].
In our study, the preparation of different experimental nanaogels; Liquorice, Propolis, and Chlorohexidine was based upon several facts like being biocompatible, having high antibacterial property, and, better penetration [25, 32,33,34,35]. In addition, Wael et al. [33] stated more advantages of the hydrogels including the controlled release in addition to the high antibacterial affinity. This was coincidence with our findings where Propolis and Chlorhexidine groups showed a statistically significant low microleakage which in turn might reflect the good performance of such nanogels as they could perfectly disinfect the prepared cavities without retarding adhesion.
Concerning microleakage results as shown in (Tables 2, 3) and Figs. (2, 3, 4, 5, 6); the negative control group had high statistically significant microleakage values at both gingival and occlusal margins. This was in accordance with several studies [36,37,38] which proved that the negative control group had high degree of microleakage at the tooth-restoration in comparison to other groups using different cavity disinfectant protocols. On the other hand, Mutluay et al. [37] and Unal et al. [36] proved that the negative control group didn’t show any significante higher degree of microleakage than other groups using different cavity disinfectant methods. This might be due to different methodologies where different bonding technique was applied as well as using different restorative materials.
While Chlorhexidine group had low statistically significant microleakage values at the tooth restoration interface which can reach zero. This was in accordance with Ramezanian et al. [38] who proved that application of Chlorhexidine cavity disinfectant significantly reduces the degree of microleakage not only immediately but also in the long term. In addition, Satpute et al. [8] review article claimed that using Chlorhexidine enhances the durability of the restorative material due to its anti-collagenolytic action on matrix metalloproteinases enzyme (MMPs). On the other hand, Mutluay et al. [37] stated that Clorhexidine did not negatively affect microleakage in the same manner as other cavity disinfectant protocols used in their study.
Moreover, Propolis group revealed high statistically significant microleakage values in the occlusal margin while the gingival margin showed less microleakage values. This might be attributed to the medium viscosity of the prepared nanogel. Up till now, there isnt any study that evaluated the effect of Propolis cavity disinfectant on microleakage, while limited studies evaluated its antibacterial effect [9, 10, 19].
Also Liquorice group showed high statistically significant microleakage values at both gingival and occlusal margins. This finding might be attributed to the high viscosity of the prepared nanogel that could prevent further penetration of the applied bond. By consequence, this could affect composite restoration sealing ability that leads to high degree of microleakage at the tooth-restoration interface. Although Liquorice cavity disinfectant has been studied for its antibacterial effect [11] but still there isn’t any available study that evaluated its sealing ability.
On the other hand, Diode laser group 980 nm had the least statistically significant microleakage values which could reach zero-degree at gingival and occlusal margin as well. This outcome reflected the ability of Diode Laser to attain a good restoration sealing quality. Unfortunately, there was a shortage of studies evaluating the effect of Diode laser as a cavity disinfectant on the degree of microleakage at tooth- restoration interface while limited studies evaluated its effect on the bond strength or its antibacterial potentiality [10, 31, 34]. In accordance with our results, Golbari et al. [39] concluded that 810 nm Diode Laser could reduce marginal microleakage at the tooth-restorations interface. However, Ipek et al. [6] proved that the Diode laser did not show any favorable effect on the drgree of microleakage at tooth—restoration interface. This might be due to different Laser parameters used in our study, as cavities were irradiated with 2 laser cycles which might enhance the adaptation of the applied restorative material.
Thus the null hypothesis is partially rejected as CHX and Propolis nanogels as well as Diode Laser have a low degree of microleakage at the tooth-restoration interface and good sealing ability while Liquorice nanogel negatively affect the sealing ability at tooth-restoration interface.
On the other hand, some limitations were found in this study as using different types of restorative materials and bonding protocols would be of value. Also long-term studies are required to evaluate the effect of different disinfection protocols on the degree of microleakage at tooth –restoration interface.
Conclusions
Within the limitation of this in vitro study, cavity disinfection using Propolis nannogel showed comparative results to nanoChloehexidine as both had a low degree of microleakage at the tooth restoration interface. Liquiorice nanogel had an obvious negative effect on the sealing ability of composite resin restoration while, Diode Laser didn’t affect its sealing ability.
Benefits of the findings
-
This study highlighted the importance of using either CHX nanogel orDiode laser cavity disinfectant protocols as they have either minimum or even absence of microleakage at the tooth-restoration interface.
-
Propolis nanogel has a comparable effect to CHX nanogel which may be attributed to the medium viscosity of the prepared nanogel that encourages its use as a cavity disinfectant.
-
Liquorice nanogel isn’t preferred as a cavity disinfectant due high degree of microleakage at the tooth-restoration interface that strongly affect the restoration sealing ability.
Data availability
The datasets of this study are available from the corresponding author upon reasonable request.
References
Askar H, Krois J, Göstemeyer G, Bottenberg P, Zero D, Banerjee A, et al. Secondary caries. What is it, and how it can be controlled, detected, and managed? J Clin Oral Investig. 2020;24:1869–76.
Singhal DK, Acharya S, Thakur AS. Microbiological analysis after complete or partial removal of carious dentin using two different techniques in primary teeth: a randomized clinical trial. Dent Res J. 2016;13:30–37.
Coelho A, Amaro I, Rascão B, Marcelino I, Paula A, Saraiva J, et al. Effect of cavity disinfectants on dentin bond strength and clinical success of composite restorations—a systematic review of in vitro, in situ and clinical studies. Int J Mol Sci. 2021;22:353.
Favetti M, Schroeder T, Montagner AF, Correa MB, Pereira-Cenci T, Cenci MS. Effectiveness of pre-treatment with chlorhexidine in restoration retention: a 36-month follow-up randomized clinical trial. J Dent. 2017;60:44–49.
Coelho A, Amaro I, Apolónio A, Paula A, Saraiva J, Ferreira MM, et al. Effect of cavity disinfectants on adhesion to primary teeth—a systematic review. Int J Mol Sci. 2021;22:4398.
Ipek A, Ozgul B, Tamer T, Fatih E, Aykut C, Mehmet KF. The effects of cavity disinfection on the nanoleakage of compomer restorations: an in vitro study. J Eur Oral Res. 2020;54:16–24.
Lacerda-Santos R, Sampaio GA, Moura MF, Carvalho FG, Santos AD, Pithon MM. Effect of different concentrations of chlorhexidine in glass-ionomer cements on in vivo biocompatibility. J Adhesive Dent. 2016;18:325–30.
Satpute TS, Mulay SA. Chlorhexidine in operative dentistry—A review. J Int Clin Dent Res Organ. 2021;13:80–85.
Neelakantan P, Jagannathan N, Nazar N. Ethnopharmacological approach in endodontic treatment: a focused review”. Int J Drug Dev Res. 2011;3:68–77.
Mohan PV, Uloopi KS, Vinay C, Rao RC. In vivo comparison of cavity disinfection efficacy with APF gel, propolis, diode laser, and 2% chlorhexidine in primary teeth. J Contemp Clin Dent. 2016;7:45–50.
Godbole E, Tyagi S, Kulkarni P, Singla S, Mali S, Helgeet S. Efficacy of liquorice and propolis extract used as cavity cleaning agents against streptococcus mutans in deciduous molars using confocal microscopy: an in vitro study. Int J Clin Pediatr Dent. 2019;12:194–200.
Lusche I, Dirk C, Frentzen M, Meister J. Cavity disinfection with a 445 nm diode laser within the scope of restorative therapy—a pilot study. J Lasers Med Sci. 2020;11:417–26.
Hou Y, Shao J, Fu Q, Li J, Sun J, He Z. Spray-dried nanocrystals for a highly hydrophobic drug: Increased drug loading, enhanced redispersity, and improved oral bioavailability. Int J Pharm. 2017;516:372–9.
Awan AM, Farid A, Shah SU, Khan D, Ur Rehman F, Dar MJ, et al. Nanocrytals-mediated oral drug delivery: enhanced bioavailability of amiodarone. Pharmaceutics. 2022;14:1300.
Sadony DM, Abozaid HE. Antibacterial effect of metallic nanoparticles on Streptococcus mutans bacterial strain with or without diode laser (970 nm). Bull J Natl Res. Cent. 2020;44:2.
Shan X, Tian F, Li J, Yang N, Wang Y, Sun H. Comparison of Er:YAG laser and ultrasonic in root canal disinfection under minimally invasive access cavity. Lasers Med Sci J. 2022;37:3249–58.
De Sousa FS, Nemezio MA, Corona SA, Aires CP, Borsatto MC. Effects of low-level laser therapy combined with toluidine blue on polysaccharides and biofilm of Streptococcus mutans. Lasers Med Sci J. 2016;31:1011–6.
Jowkar Z, Farpour N, Koohpeima F, Mokhtari MJ, Shafiei F. Effect of silver nanoparticles, zinc oxide nanoparticles and itanium dioxide nanoparticles on microshear bond strength to enamel and dentin. J Contemp Dent Pract. 2018;19:1405–12.
Bin-Shuwaish MS. Effects effectiveness of cavity disinfectants in operative dentistry: a literature review. J Contemp Dent Pract. 2016;17:867–79.
Rathi SD, Nikhade P, Chandak M, Motwani N, Rathi C, Chandak M. Microleakage in composite resin restoration- a review article. J Evolut Med Dent Sci. 2020;9:1006–11.
Bin‑Shuwaish M, AlHussaini A, AlHudaithy L, AlDukhiel S, AlJamhan A, Alrahlah A. Effects of different antibacterial disinfectants on microleakage of bulk‑fill composite bonded to different tooth structures. BMC Oral Health. 2021;21:348.
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6.
Essa E, Negm M, Eldin EZ, El Maghraby G. Fast disintegrating tablets of amiodarone for intra-oral administration. J Appl Pharmcut Sci. 2017;7:72–072.
Willers AE, Ayres AP, Hirata R, Giannini M. Effect of universal adhesive application on bond strength of four-year aged composite repair. J Adhes Sci Technol. 2022;36:1823–32.
Nassaja AE, Ghadimib S, Serajc B, Chiniforush N. Effect of photodynamic therapy on microleakage of class V composite restorations in primary teeth. J Photodiagnosis Photodyn Ther. 2020;32:101964.
AlHadad M, AlGharrawi H, AlHashemi J. Microleakage evaluation of sonicfill,silorane-based and nanofilled methacrylate based composites .“(a comparative study). JODR. 2017;4:120–9.
Parco TM, Tantbirojn D, Versluis A, Beiraghi S. Microleakage of self-etching sealants on dry and saliva contaminated enamel. J Pediatr Dent. 2011;33:479–83.
Hosseinipour ZS, Heidari A, Shahrabi M, Poorzandpoush K. Microleakage of a self-adhesive flowable composite, a self-adhesive fissure sealant and a conventional fissure sealant in permanent teeth with/without saliva contamination. Front Dent J. 2019;16:239–47.
Celik C, Özel Y, Bagis B, Erkut S. Effect of laser irradiation and cavity disinfectant application on the microtensile bond strength of different adhesive systems. Photomed Laser Surg. 2010;28:267–72.
Salama FS, Balto H, Al-Yahya F, Al-Mofareh S. Effect of cavity disinfectants on microleakage of composite restorations in primary teeth. Eur J Paediatr Dent. 2015;16:295–300.
Arslan I, Baygin O, Bayramoglu G, Akyol R, Tuzuner T. Effects of various agents and laser systems on antibacterial activity and microtensile bond strength when used for cavity disinfection. J Dent Lasers. 2019;13:12–8.
Zarekar NS, Lingayat VJ, Pande VV. Nanogel as a novel platform for smart drug delivery system. Nanosci Nanotechnol Res J. 2017;4:25–31.
Wael HM, Widad KA, Mohammed JA. Evaluation of in vitro drug release kinetics and antibacterial activity of vancomycin HCl loaded nanogel for topical application. J Pharm Sci Res. 2018;10:2747–56.
Jain S, Mathur S, Jhingan P, Sachdev V. Evaluation of temperature rise and efficacy of cavity disinfection with diode laser: an in vivo study. J. Conserv Dent. 2019;22:583–7.
Saleh RS, Nagi SM, Elgamily HM, Hassan SN, El-Hashemy H, Zaazou MH. Evaluation of the antibacterial activity and the remineralizing potentiality of two natural extracts and their mixture (in vitro study). Macedonian J Med Sci. 2022;10:116–23.
Unal S, Akbiyik SY, Bakir EP, Bakir S. Effect of different cavity disinfectants on microleakage. J Evolut Med Dent Sci. 2021;10:3752–6.
Mutluay AT, Mutluay M. Effects of different disinfection methods on microleakage of giomer restorations. Eur J Dent. 2019;13:569–73.
Ramezanian I, Baradaran E, Majidinia S, Ramezanian S, Jafari M. Effect of chlorhexidine and ethanol on microleakage of composite resin restoration to dentine. Chin J Dent Res. 2017;20:161–8.
Golbari N, Kasraei S, Afrasiabi A, Mostajir E, Mojahedi SM. Effect of diode laser (810 nm) irradiation on marginal microleakage of multi-mode adhesive resins in class V composite restorations. J Lasers Med Sci. 2019;10:275–82.
Funding
This research was self-funded. Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MMM: study design, data assessment, experiments performance, and Funding. SST: experiment performance, data collection and funding. RSS: writing, data analysis, and manuscript writing. RA: material formulation, writing, data analysis, and manuscript review. HM: study design, data assessment, final manuscript approval. NS: study design, data collection, and interference.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This in vitro study was approved by the Institutional Review Board at Misr University for Science and Technology (MUST- IRB) under number: 2022/0066.
Consent to participate
Consent to participate was not needed. Teeth were extracted from orthodontic patients in MUST university clinic where the patients were informed that their teeth will be used for research purposes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ElMansy, M.M., Tadros, S.S.T., Saleh, R.S. et al. Comparative evaluation on the effect of different cavity disinfectant nano gels; Chlorohexidine, Propolis, Liquorice versus Diode Laser in terms of composite microleakage (comparative in vitro study). BDJ Open 9, 49 (2023). https://doi.org/10.1038/s41405-023-00176-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-023-00176-2