Abstract
Introduction
Fibrocartilaginous embolism (FCE) of the spinal cord is an ischemic myelopathy caused by rupture of the intervertebral disc and subsequent entrance of the nucleus polposus material into the nearby vasculature. However, responsible disc lesions frequently cannot be determined, which may cause diagnostic difficulty.
Case presentation
A 63-year-old man suddenly felt a strange sensation in the lower limbs and noticed that he could not walk. The patient was brought to our hospital, where he was hospitalized. On neurological examination, his proprioception and vibratory sense were disturbed in the lower limbs. Spine magnetic resonance imaging (MRI) revealed a hyperintense cord lesion located at the C4–5 vertebral level of the posterior spinal artery region on diffusion-weighted sequence. He was diagnosed as having spinal cord infarction and treated with antiplatelet medication. Follow-up MRI was performed 5 days later, which displayed a collapsed C3/4 disc and a defected C4 vertebral body that were not evident on earlier MRI. These findings suggested the disc lesion was active and responsible for the infarction, leading to the diagnosis of FCE. The patient gradually improved and had no gait difficulty at the time of discharge.
Discussion
In the presented case, MRI did not reveal the responsible disc lesion when the diagnosis of spinal cord infarction was made; however, it became evident 5 days later. Although such a disc signal change within several days has not been described as a clue suggesting FCE, follow-up MRI with a relatively short interval may elucidate the responsible lesion and correct diagnosis.
Similar content being viewed by others
Introduction
Fibrocartilaginous embolism (FCE) of the spinal cord is a seemingly rare ischemic myelopathy that probably tends to be overlooked in clinical practice because of the diagnostic difficulty [1]. Typically, in patients with this disease, the symptoms are preceded by heavy lifting, minor trauma, or any event that presses the intervertebral disc. The increased pressure causes rupture of the disc and entrance of the nucleus polposus material into the nearby vasculature, followed by migration to the spinal cord and embolization [2,3,4].
In this type of ischemic myelopathy, the presence of disc pathology such as collapses, horizontal herniations, or vertical herniations (Schmorl’s nodes) located near the infarcted cord is theoretically essential. These disc lesions can be found frequently on magnetic resonance imaging (MRI) [5,6,7]; however, they are too common in adults and multiple disc lesions may be detected in a patient. Therefore, it is often unclear whether pathological discs on MRI that exist near the cord lesion are truly the cause because of the possibility that they are simply located there and unrelated to the infarction.
Spinal cord infarction caused by FCE usually involves the region of the anterior spinal artery (ASA), although some reports described patients with ischemia in the posterior spinal artery (PSA) region [8]. PSA infarction is rare and occasionally manifests as ataxic gait without apparent muscle weakness; hence, it might require more attention than ASA infarction for correct diagnosis [9, 10]. Here, we present the case of a patient with spinal cord FCE, in whom follow-up MRI detected a collapsed intervertebral disc that existed near an ischemic cord lesion and was not evident on earlier MRI. Moreover, the infarction was located in the PSA region.
Case presentation
A 63-year-old man suddenly felt a strange sensation in the lower limbs and noticed that he could not walk. He initially rested at home for several hours but was brought to our hospital because the symptoms remained the same. His medical history included hypertension and diabetes mellitus, and he took medications such as candesartan 4 mg/day, glimepiride 2 mg/day, dapagliflozin 10 mg/day, and metformin 1 mg/day. His blood pressure was 142/70 mmHg. On neurological examination, the patient had normal limb muscle strength with preserved pain sensation; however, his proprioception and vibratory sense in the lower limbs were disturbed with a left predominance and Romberg sign was positive. He was hospitalized due to the standing and gait difficulty.
On the day of symptom onset, MRI of the head and spine was performed, but no responsible lesion was detected. Although diffusion-weighted sequence was included for the head, it was not for the spine. Magnetic resonance angiography of the head and neck displayed no abnormalities. In the routine blood tests, serum uric acid (7.3 mg/dl; normal range, 3.7–7.0 mg/dl) and blood glucose (160 mg/dl, 73–109 mg/dl) were elevated, with normal levels of prothrombin time, activated partial prothrombin time, and D-dimer. Chest X-ray and electrocardiogram were unremarkable.
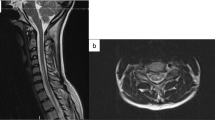
Spine MRI was reperformed 4 days after symptom onset. Diffusion-weighted sequence revealed a hyperintensity cord lesion at the C4–5 vertebral level (arrow, Fig. 1a), which was hypointense on ADC map (arrow, Fig. 1b) and located in the PSA region (arrow, Fig. 2a). The cord lesion was not apparent on T1 (Fig. 1c) and displayed as a linear slight hyperintensity on T2 (horizontal arrow, Fig. 1d). The linear T2 hyperintensity was not evident on the initial MRI. A triangular abnormality in the anterior part of the C4 vertebral body (oblique arrows, Fig. 1c, d), a horizontal disc herniation at the C4/5, and decreased disc height at the C6/7 were also observed. Cerebrospinal fluid analysis exhibited normal cell counts and glucose concentration with elevated total protein (83 mg/dl; normal range, 10–50 mg/dl). In additional blood tests, serum vitamin B12, anti-nuclear antibody, anti-SSA and SSB antibodies, protein C and S activity, lupus anticoagulant, and anticardiolipin and anti-beta 2 glycoprotein I antibodies were nondiagnostic. Neither aortic dissection nor thrombus was found on dynamic computed tomography. The sudden onset symptoms without following deterioration, the T2 hyperintensity lesion in the PSA region that was not evident on the initial MRI, and no evidence of inflammation in the cerebrospinal fluid suggested that he had spinal cord infarction [3, 11,12,13]. The abnormal hyperintensity on diffusion-weighted sequence also contributed to the diagnosis [13,14,15]. Oral clopidogrel 75 mg/day was started; intravenous treatments specific for spinal cord infarction were not administered because his symptoms gradually improved after hospitalization.
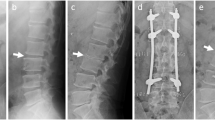
Diffusion-weighted sequence obtained 4 days after symptom onset revealed a linear hyperintensity cord lesion at the C4–5 vertebral level (arrow, a). The lesion was hypointense on ADC map (arrow, b). A triangular T1 hypointense abnormality existed in the anterior part of the C4 vertebral body (arrow, c), which displayed irregular intensities on T2 (oblique arrow, d). The cord lesion was slightly hyperintense on T2 (horizontal arrow, d). On follow-up MRI 5 days later, a part of the triangular lesion on T1 (arrow, e) and T2 (arrow, f) became defected. The defect was less evident 45 days after symptom onset (arrows, g, h; T1 and T2 images, respectively).
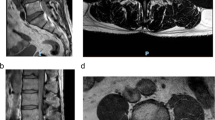
Diffusion-weighted sequence performed 4 days after symptom onset exhibited a hyperintensity lesion in the posterior part of the spinal cord (arrow, a). On T2-sequence MRI 5 days later, the lesion was mainly located in the left-sided posterior column (arrow, b). Panels c–e display T1 (upper columns) and T2 (lower) signal changes of the C3/4 intervertebral disc and C4 vertebral body 4 (c), 9 (d), and 45 (e) days after symptom onset. The anterior part of the C3/4 disc was transiently collapsed (arrows, d).
Follow-up MRI was performed 9 days after symptom onset. On axial T2 image at the C4/5 level, the infarct was mainly located in the left-sided PSA region (arrow, Fig. 2b). In comparison with the C3/4 disc finding 4 days after symptom onset (Fig. 2c), the anterior part of the disc became collapsed (arrows, Fig. 2d). Moreover, a part of the triangular lesion was defected (arrows, Fig. 1e, f; Fig. 2d). These findings suggested that lesions in the C3/4 disc and C4 vertebral body were active and related to the infarction, which prompted us to obtain a more detailed anamnesis. In fact, the patient was a shop clerk and often lifted heavy goods while working. Before 6 h from the symptom onset, as usual he raised corrugated cardboard boxes, each of which contained pet bottle drinks and weighed approximately 12 kg, and placed them on the shelf. The chronological signal changes in the disc and vertebral body and episodes of heavy lifting suggested that the spinal cord infarction was caused by FCE [3]. The patient gradually recovered and became able to stand and gait without difficulty, and then he was discharged. During the patient’s subsequent visit to our outpatient clinic, spinal MRI was performed again 45 days after the onset of symptoms. The infarct remained as an area of T2 hyperintensity at the same location, while the lesions in the disc and vertebral body were less evident (arrows, Fig. 1g, h; Fig. 2e).
Discussion
In the presented case, MRI obtained 4 days after symptom onset indicated acute infarction although the C3/4 intervertebral disc lesion was not apparent; in contrast, MRI 9 days after symptom onset displayed the collapsed disc and defect in the C4 vertebral body. Moreover, the infarct was located in the PSA region.
In the antemortem diagnosis of spinal cord FCE, abnormal disc findings on MRI are considered of importance. Patients with FCE were often reported to have degenerative disc changes on MRI [5,6,7, 16]; however, chronological signal changes of the disc within several days have not been described as findings that may indicate responsible lesions. In our case, the C4/5 and 6/7 disc lesions were apparent on MRI when the diagnosis of spinal cord infarction was made but they did not reveal chronological changes. Collapse of the C3/4 disc was not found when MRI initially detected the spinal cord infarction, which became evident 5 days later. Moreover, the collapsed disc was restored along with the healing of the C4 vertebral body.
Although the exact mechanism of these disc changes is not clear, hemorrhage from the C4 vertebral body could have caused the changes. The triangular lesion of the C4 vertebral body 4 days after the onset of symptoms indicated acute fracture because of the following bone defect and was probably filled with blood and fluid [17]. As the fractured vertebral body was contiguous to the anterior C3/4 disc, the anterior disc is considered to have been injured together and similarly filled with the substances from the fractured bone. Regarding the disc collapse, one possibility is that the substances pressed the injured disc posteriorly. Another possibility is that the blood and fluid made magnetic resonance signals of the disc ambiguous. These possibilities are compatible with the restoration of the C3/4 disc because these substances are inferred to have regressed along with the healing of the C4 vertebral body. The C3/4 disc lesion was likely responsible for the infarction as its chronological signal change indicated recent damage to the disc. In patients who have suspected spinal cord FCE, follow-up MRI should be performed with a relatively short interval to detect chronological changes of the intervertebral disc.
The presented patient had an infarct in the PSA region, although most reported cases had ischemic lesions in the ASA region. According to a major hypothesis, excessive axial loading to the spine, which is induced by various events such as heavy lifting, minor trauma, and physical exercise, presses the disc and causes its rupture. Then the nucleus polposus material penetrates the vertebral endplate and protrudes to the vertebral body, resulting in the entrance of the material into the arterial system and retrograde migration in the supplying artery of the vertebral body [4]. In the cervical region, the vertebral artery often gives off single branches that subdivide into multiple vessels supplying the vertebral body, spinal cord, and paravertebral muscles [18]. Therefore, after the retrograde migration, the material can move anterogradely into the spinal cord [4]. When the branch of the vertebral artery into which the migrated material moves has anastomoses with the ASA, infarcts in the anterior two-thirds of the cord may occur. In the case of the branch that has connections with the PSA, infarction might be located in the posterior column although it is rare, probably because of abundant arterial network collaterals [9, 10]. In the presented case, the branch into which the nucleus polposus material migrated supposedly had connections with the PSA.
There are additional considerations related to the diagnosis and treatment. Although this patient met the diagnostic criteria for FCE [3], the possibility of thrombotic infarction was not completely ruled out as the diagnosis of FCE was not confirmed pathologically. The patient’s symptoms gradually improved after hospitalization and no effective treatment was established for spontaneous spinal cord infarction. Thus specific intravenous drugs were not used in this case. If his symptoms had progressed with cord edema, however, he could have been treated with intravenous glycerol. Moreover, intravenous heparin might have been administered in the presence of a responsible aortic thrombus.
The diagnosis of spinal cord FCE is often difficult possibly because the responsible intervertebral disc cannot be determined definitively. Follow-up MRI performed with a relatively short interval may contribute to the diagnosis of FCE when chronological changes in the disc are observed. Moreover, spinal cord infarction due to FCE can involve the PSA region.
References
Moore BJ, Batterson AM, Luetmer MT, Reeves RK. Fibrocartilaginous embolic myelopathy: Demographics, clinical presentation, and functional outcomes. Spinal Cord. 2018;56:1144–50.
Mateen FJ, Monrad PA, Hunderfund AN, Robertson CE, Sorenson EJ. Clinically suspected fibrocartilaginous embolism: Clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218–25.
AbdelRazek MA, Mowla A, Farooq S, Silvestri N, Sawyer R, Wolfe G, et al. Fibrocartilaginous embolism: A comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. J Spinal Cord Med. 2016;39:146–54.
Tosi L, Rigoli G, Beltramello A. Fibrocartilaginous embolism of the spinal cord: A clinical and pathogenetic reconsideration. J Neurol Neurosurg Psychiatry. 1996;60:55–60.
Davis GA, Klug GL. Acute-onset nontraumatic paraplegia in childhood: Fibrocartilaginous embolism or acute myelitis? Childs Nerv Syst. 2000;16:551–4.
Raghavan A, Onikul E, Ryan MM, Prelog K, Taranath A, Chennapragada M, et al. Anterior spinal cord infarction owing to possible fibrocartilaginous embolism. Pediatr Radio. 2004;34:503–6.
Duprez TP, Danvoye L, Hernalsteen D, Cosnard G, Sindic CJ, Godfraind C, et al. Fibrocartilaginous embolization to the spinal cord: Serial MR imaging monitoring and pathologic study. AJNR Am J Neuroradiol. 2005;26:496–501.
Bansal S, Brown W, Dayal A, Carpenter JL. Posterior spinal cord infarction due to fibrocartilaginous embolization in a 16-year-old athlete. Pediatrics. 2014;134:e289–92.
Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology. 2002;44:851–7.
Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: Aaetiology, clinical syndromes and imaging features. Neuroradiology. 2015;57:241–57.
Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: Clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113–20.
Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis. 2009;27:493–501.
Zalewski NL, Rabinstein AA, Krecke KN, Brown RD Jr, Wijdicks EFM, Weinshenker BG, et al. Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76:56–63.
Kuek DKC, Chung SL, Zishan US, Papanikitas J, Yanny S, Meagher T, et al. Conus infarction after non-guided transcoccygeal ganglion impar block using particulate steroid for chronic coccydynia. Spinal Cord Ser Cases. 2019;5:92.
Morshid A, Jadiry HA, Chaudhry U, Raghuram K. Pediatric spinal cord infarction following a minor trauma: A case report. Spinal Cord Ser Cases. 2020;6:95.
Draganich C, Wenzel LR. Fibrocartilagenous embolism case series: Is it a zebra? Spinal Cord Ser Cases. 2021;7:28.
Griffith JF, Guglielmi G. Vertebral fracture. Radio Clin North Am. 2010;48:519–29.
Ozgen S, Pait TG, Cağlar YS. The V2 segment of the vertebral artery and its branches. J Neurosurg Spine. 2004;1:299–305.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kobayashi, M. Fibrocartilaginous embolism of the posterior spinal artery: A case report regarding the responsible intervertebral disc on magnetic resonance imaging. Spinal Cord Ser Cases 8, 10 (2022). https://doi.org/10.1038/s41394-022-00477-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00477-y