Abstract
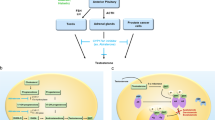
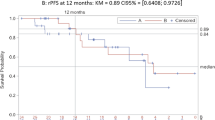
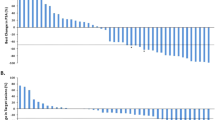
Combined androgen deprivation therapy (ADT) and radiotherapy (RT) improves outcomes for intermediate and high-risk prostate cancer. Treatment intensification with abiraterone acetate/prednisone (AAP) provides additional benefit for high-risk disease. We previously reported 3-year outcomes of a single-arm prospective multicenter trial (AbiRT trial) of 33 patients with unfavorable intermediate risk (UIR) and favorable high risk (FHR) prostate cancer undergoing short course, combination therapy with ADT, AAP, and RT. Here we report the final analysis demonstrating a high rate of testosterone recovery (97%) and excellent biochemical progression-free survival (97%) at 5 years. These data support comparative prospective studies of shorter, more potent ADT courses in favorable high-risk prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bolla M, Maingon P, Carrie C, Villa S, Kitsios P, Poortmans PMP, et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC trial 22991. J Clin Oncol. 2016;34:1748–56.
Krauss DJ, Karrison T, Martinez AA, Morton G, Yan D, Bruner DW, et al. Dose-escalated radiotherapy alone or in combination with short-term androgen deprivation for intermediate-risk prostate cancer: results of a phase III multi-institutional trial. J Clin Oncol. 2023. https://doi.org/10.1200/JCO.22.02390.
Lawton CAF, Lin X, Hanks GE, Lepor H, Grignon DJ, Brereton HD, et al. Duration of androgen deprivation in locally advanced prostate cancer: long-term update of NRG Oncology RTOG 9202. Int J Radiat Oncol Biol Phys. 2017;98:296–303.
Nabid A, Carrier N, Martin A-G, Bahary J-P, Lemaire C, Vass S, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur Urol. 2018;74:432–41.
Nabid A, Carrier N, Martin A-G, Vigneault E, Vincent F, Brassard M-A, et al. Testosterone recovery in patients with prostate cancer treated with radiotherapy and different ADT duration: long-term data from two randomized trials. J Clin Oncol. 2023;41:300.
Koontz BF, Hoffman KE, Halabi S, Healy P, Anand M, George DJ, et al. Combination of radiation therapy and short-term androgen blockade with abiraterone acetate plus prednisone for men with high- and intermediate-risk localized prostate cancer. Int J Radiat Oncol Biol Phys. 2021;109:1271–8.
Bolla M, De Reijke TM, Van Tienhoven G, Van Den Bergh ACM, Oddens J, Poortmans PMP, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27.
Armstrong AJ, Liu VY, Selvaraju RR, Chen E, Simko J, DeVries S, et al. Development and validation of an AI-derived digital pathology-based biomarker to predict benefit of long-term androgen deprivation therapy with radiotherapy in men with localized high-risk prostate cancer across multiple phase III NRG/RTOG trials. J Clin Oncol. 2023;41:5001.
Nguyen PL, Kollmeier M, Rathkopf DE, Hoffman KE, Zurita AJ, Spratt DE, et al. FORMULA-509: a multicenter randomized trial of post-operative salvage radiotherapy (SRT) and 6 months of GnRH agonist with or without abiraterone acetate/prednisone (AAP) and apalutamide (Apa) post-radical prostatectomy (RP). J Clin Oncol. 2023;41:303.
Acknowledgements
The study was conducted within the US Department of Defense (DOD) Prostate Cancer Clinical Trials Consortium with institutional review board approval through participating sites: Duke Cancer Institute and MD Anderson Cancer Center (ClinicalTrials.gov NCT01717053). This work was supported by funding from Janssen Scientific Affairs. The infrastructure to complete this trial was supported by the 2 consecutive Department of Defense Congressionally Directed Medical Research Program (DOD CDMRP) Prostate Cancer Clinical Trials Consortium grants: W81XWH-09-1-0152 and W81XWH-14-2-0198.
Author information
Authors and Affiliations
Contributions
REF: data interpretation, manuscript writing and editing. BFK: trial design, data interpretation, trial enrollment, manuscript editing. KEH: data interpretation, trial enrollment, manuscript editing. SH: statistical analysis, data interpretation, manuscript editing. LEH: statistical analysis, data interpretation, manuscript editing. MA: statistical analysis, data interpretation. DJG: trial design, data interpretation, trial enrollment. TZ: trial design, data interpretation, trial enrollment. WRB: trial design, trial enrollment. WRL: data interpretation, trial enrollment, manuscript editing. MRH: data interpretation, trial enrollment. PGC: data interpretation, trial enrollment. AJA: trial design, data interpretation, trial enrollment, manuscript writing and editing.
Corresponding author
Ethics declarations
Competing interests
REF has received consulting fees from Decipher Biosciences, Eisai, Seagen/Pfizer, and participated in advisory boards for Telix. BFK received research funding (to Duke) from Janssen Scientific Affairs for this work. She also has served on advisory boards for Blue Earth Diagnostics, Myovant, and Merck, and is a member of the board of directors for Rythera Therapeutics. KEH: Research Funding (to my institution from): Varian Medical Systems, Rising Tide Foundation, Janssen Scientific Affairs. SH: Member of DSMB for Aveo, BMS, J&J, Sanofi. LEH: none. MA: none. DJG has research support (to Duke University) from Acerta, Astellas, BMS, Bayer, Calithera, Exelixis, Janssen, Myovant, Pfizer, Novartis, and Sanofi Aventis, and consulting income from Vizuri Health Sciences, UroToday, Sanofi, Pfizer, Nektar, Myovant, Modra, Merck, Ipsen, Flatiron, Exelixis, Capio, EMD Serono, BMS, Bayer, AstraZeneca, and Astellas. TZ has received research funding (to Duke) from Pfizer, Janssen, Acerta, AbbVie, Novartis, Merrimack, OmniSeq, PGDx, Merck, Mirati, Astellas, and Regeneron; consulting and speaking income from Genentech Roche, Exelixis, Genomic Health, and Sanofi Aventis; has received consulting income from AstraZeneca, Bayer, Pfizer, Foundation Medicine, Janssen, Amgen, MJH Associates, Merck, BMS, Pharmacyclics, and Seattle Genetics. WRB: none. WRL: none. MRH has research funding (to Duke University) from Argos, Bayer, BMS, Exelixis, Pfizer, Merck, and Seattle Genetics and has received consulting and speaking income from BMS, Genentech Roche, Exelixis, and Janssen. PGC receives institutional funding support from Janssen. AJA: Research support (to Duke) from the NIH/NCI, PCF/Movember, DOD, Astellas, Pfizer, Bayer, Janssen, Dendreon, BMS, AstraZeneca, Merck, Forma, Celgene, Amgen, Novartis. Consulting or advising relationships with Astellas, Epic Sciences, Pfizer, Bayer, Janssen, Dendreon, BMS, AstraZeneca, Merck, Forma, Celgene, Clovis, Exact Sciences, Myovant, Exelixis, GoodRx, Novartis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fecteau, R.E., Koontz, B.F., Hoffman, K.E. et al. Updated 5-year results for short course abiraterone acetate and LHRH agonist for unfavorable intermediate and favorable high-risk prostate cancer. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00811-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00811-5