Abstract
Background
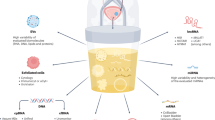
Current diagnostic methods for prostate cancer are invasive and lack specificity towards aggressive forms of the disease, which can lead to overtreatment. A new class of non-invasive alternatives is under development, in which urinary biomarkers are detected using biosensing devices to offer rapid and accurate prostate cancer diagnosis. These different approaches are systematically reviewed and their potential for translation to clinical practice is evaluated.
Methods
A systematic review of the literature was performed in May 2021 using PubMed Medline database, Embase, and Web of Science. The objective was to review the structural designs and performance of biosensors tested on urine samples from patients with prostate cancer.
Results
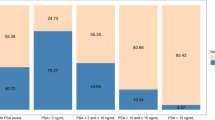
A total of 76 records were identified. After screening and eligibility, 14 articles were included and are discussed in this paper. The biosensors were discussed based on the target biomarkers and detection technologies used, as well as the results of the clinical studies. Most of the works reported good discrimination between patients with prostate cancer and controls.
Conclusions
This review highlights the potential of urinary biosensors for non-invasive prostate cancer detection. However, clinical studies have so far only been conducted on small cohorts of patient, with large scale trials still needed to validate the proposed approaches. Overall, the consensus arising from the proof of concepts studies reviewed here, is that an adequate combination of biomarkers into multiplex biosensor platforms is required to achieve accurate diagnostic tests. Furthermore, whether such devices can discriminate between aggressive and indolent cancer has not yet been addressed, because it entails optimized biomarkers panels and long-term clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferlay JEM, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020 2021. https://gco.iarc.fr/today.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer J clinicians 2020;70:7–30.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2021;79:243–62.
Carroll PR, Parsons JK, Andriole G, Bahnson RR, Barocas DA, Castle EP, et al. NCCN clinical practice guidelines prostate cancer early detection, Version 2.2015. J Natl Compr Cancer Netw: Jnccn 2015;13:1534–61.
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013;14:1165–74.
Gustavsen G, Gullet L, Cole D, Lewine N, Bishoff JT. Economic burden of illness associated with localized prostate cancer in the United States. Future Oncol 2020;16:4265–77.
Wood SL, Knowles MA, Thompson D, Selby PJ, Banks RE. Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat Rev Urol 2013;10:206–18.
MacGregor M, Safizadeh Shirazi H, Chan KM, Ostrikov K, McNicholas K, Jay A, et al. Cancer cell detection device for the diagnosis of bladder cancer from urine. Biosens Bioelectron 2020;171:112699.
Chan KM, Gleadle JM, Gregory PA, Phillips CA, Shirazi HS, Whiteley A, et al. Selective microfluidic capture and detection of prostate cancer cells from urine without digital rectal examination. Cancers 2021;13:5544.
Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan DW, et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol 2017;3:1085–93.
Haese A, Trooskens G, Steyaert S, Hessels D, Brawer M, Vlaeminck-Guillem V, et al. Multicenter optimization and validation of a 2-gene mRNA urine test for detection of clinically significant prostate cancer before initial prostate biopsy. J Urol 2019;202:256–63.
McKiernan J, Donovan MJ, Margolis E, Partin A, Carter B, Brown G, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2-10ng/ml at initial biopsy. Eur Urol 2018;74:731–8.
Progensa PCA3 Assay. 2019 2021. https://www.hologic.com/package-inserts/diagnostic-products/progensa-pca3-assay.
Whole blood PSA analyzer. Claros Diagnostics 2021. https://www.eclipsepd.com/areas/life-sciences.
iBreastExam. UE LifeSciences Inc. 2021. https://www.ibreastexam.com/.
cobas h 232 POC system. F. Hoffmann-La Roche Ltd 2021. https://diagnostics.roche.com/global/en/products/instruments/cobas-h-232.html.
Afinion 2 analyzer. Abbott 2021. https://www.globalpointofcare.abbott/en/product-details/afinion2-analyzer.html.
Schmidt K, Podmore I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J Biomark 2015;2015:981458.
Hanna GB, Boshier PR, Markar SR, Romano A. Accuracy and methodologic challenges of volatile organic compound-based exhaled breath tests for cancer diagnosis: a systematic review and meta-analysis. JAMA Oncol 2019;5:e182815-e.
Guadagni R, Miraglia N, Simonelli A, Silvestre A, Lamberti M, Feola D, et al. Solid-phase microextraction–gas chromatography–mass spectrometry method validation for the determination of endogenous substances: Urinary hexanal and heptanal as lung tumor biomarkers. Analytica Chim acta 2011;701:29–36.
Aggio RBM, de Lacy Costello B, White P, Khalid T, Ratcliffe NM, Persad R, et al. The use of a gas chromatography-sensor system combined with advanced statistical methods, towards the diagnosis of urological malignancies. J Breath Res 2016;10:017106.
Cornu J-N, Cancel-Tassin G, Ondet V, Girardet C, Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: a step forward in early diagnosis. Eur Urol 2011;59:197–201.
Guest C, Harris R, Sfanos KS, Shrestha E, Partin AW, Trock B, et al. Feasibility of integrating canine olfaction with chemical and microbial profiling of urine to detect lethal prostate cancer. PLoS One 2021;16:e0245530.
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009;457:910–4.
Khan AP, Rajendiran TM, Bushra A, Asangani IA, Athanikar JN, Yocum AK, et al. The role of sarcosine metabolism in prostate cancer progression. Neoplasia (N. Y, NY) 2013;15:491–501.
Li J, Ma J, Zhang Y, Zhang Z, He G. An amperometric biosensor for the assay of sarcosine based on the cross coupled chemical and electrochemical reactions with practical applications. J Electroanalytical Chem 2019;833:568–72.
Phyo JB, Woo A, Yu HJ, Lim K, Cho BH, Jung HS, et al. Label-free SERS analysis of urine using a 3D-stacked AgNW-glass fiber filter sensor for the diagnosis of pancreatic cancer and prostate cancer. Anal Chem 2021;93:3778–85.
Solovieva S, Karnaukh M, Panchuk V, Andreev E, Kartsova L, Bessonova E, et al. Potentiometric multisensor system as a possible simple tool for non-invasive prostate cancer diagnostics through urine analysis. Sens Actuators B-Chem 2019;289:42–7.
Truong Q, Justiniano IO, Nocon AL, Soon JT, Wissmueller S, Campbell DH, et al. Glypican-1 as a biomarker for prostate cancer: isolation and characterization. J Cancer 2016;7:1002–9.
Campbell DH, Lund ME, Nocon AL, Cozzi PJ, Frydenberg M, De Souza P, et al. Detection of glypican-1 (GPC-1) expression in urine cell sediments in prostate cancer. PLoS One 2018;13:e0196017.
Rzhevskiy AS, Razavi Bazaz S, Ding L, Kapitannikova A, Sayyadi N, Campbell D, et al. Rapid and label-free isolation of tumour cells from the urine of patients with localised prostate cancer using inertial microfluidics. Cancers (Basel) 2019;12:81.
Atlas HP Human Protein Atlas. 2021 2021. http://www.proteinatlas.org.
Köllermann J, Schlomm T, Bang H, Schwall GP, von Eichel-Streiber C, Simon R, et al. Expression and prognostic relevance of annexin A3 in prostate cancer. Eur Urol 2008;54:1314–23.
Hamelin-Peyron C, Vlaeminck-Guillem V, Haïdous H, Schwall GP, Poznanović S, Gorius-Gallet E, et al. Prostate cancer biomarker annexin A3 detected in urines obtained following digital rectal examination presents antigenic variability. Clin Biochem 2014;47:901–8.
Jeun M, Park S, Kim Y, Choi J, Song SH, Jeong IG, et al. Self-normalized detection of ANXA3 from untreated urine of prostate cancer patients without digital rectal examination. Adv Healthc Mater 2017;6:1700449.
Tsai CH, Chen YT, Chang YH, Hsueh C, Liu CY, Chang YS, et al. Systematic verification of bladder cancer-associated tissue protein biomarker candidates in clinical urine specimens. Oncotarget 2018;9:30731–47.
Kim H, Park S, Jeong IG, Song SH, Jeong Y, Kim CS, et al. Noninvasive precision screening of prostate cancer by urinary multimarker sensor and artificial intelligence analysis. ACS nano 2020;15:4054–65.
Chiou CC, Chang PY, Chan EC, Wu TL, Tsao KC, Wu JT. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin Chim Acta 2003;334:87–94.
Zitka O, Krizkova S, Krejcova L, Hynek D, Gumulec J, Masarik M, et al. Microfluidic tool based on the antibody-modified paramagnetic particles for detection of 8-hydroxy-2′-deoxyguanosine in urine of prostate cancer patients. Electrophoresis 2011;32:3207–20.
Laxman B, Tomlins SA, Mehra R, Morris DS, Wang L, Helgeson BE, et al. Noninvasive detection of TMPRSS2:ERG fusion transcripts in the urine of men with prostate cancer. Neoplasia (N. Y, NY) 2006;8:885–8.
Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, Cornel EB, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol 2014;65:534–42.
Koo KM, Wee EJH, Trau M. High-speed biosensing strategy for non-invasive profiling of multiple cancer fusion genes in urine. Biosens Bioelectron 2017;89:715–20.
Koo KM, Carrascosa LG, Trau M. DNA-directed assembly of copper nanoblocks with inbuilt fluorescent and electrochemical properties: application in simultaneous amplification-free analysis of multiple RNA species. Nano Res 2018;11:940–52.
Koo KM, Dey S, Trau M. Amplification-free multi-RNA-type profiling for cancer risk stratification via alternating current electrohydrodynamic nanomixing. Small (Weinh der Bergstr, Ger) 2018;14:e1704025.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373–83.
Yasui T, Yanagida T, Ito S, Konakade Y, Takeshita D, Naganawa T, et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci Adv 2017;3:e1701133.
Woo HK, Park J, Ku JY, Lee CH, Sunkara V, Ha HK, et al. Urine-based liquid biopsy: non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab a chip 2019;19:87–97.
Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res 2014;20:3198.
Martin SK, Banuelos CA, Sadar MD, Kyprianou N. N-terminal targeting of androgen receptor variant enhances response of castration resistant prostate cancer to taxane chemotherapy. Mol Oncol 2014;9:628–39.
Author information
Authors and Affiliations
Contributions
Conceptualization, investigation, results interpretation, writing – original draft preparation, KMC and MM; contribution to an oversight of the overall study, JMG and MO; writing – review and editing, MM, JMG, MO and KV; supervision, project administration, MM, JMG and KV All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chan, K.M., Gleadle, J.M., O’Callaghan, M. et al. Prostate cancer detection: a systematic review of urinary biosensors. Prostate Cancer Prostatic Dis 25, 39–46 (2022). https://doi.org/10.1038/s41391-021-00480-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00480-8
This article is cited by
-
ExoDx test for prostate cancer: the future is liquid—Editorial Comment
Prostate Cancer and Prostatic Diseases (2023)