Abstract
Background
Several studies have discovered an association between infant feeding practices and puberty timing; however, most have involved female cohorts. We investigated the association between infant feeding practices and the timing of peak height velocity in boys and girls.
Methods
Data on infant feeding methods and anthropometric measurements were collected from a nationwide Japanese birth cohort study. The age at peak height velocity (APV, years) was estimated and compared. Subsequently, the effects of breastfeeding duration were analyzed.
Results
Of the 13,074 eligible participants, 650, 9455, and 2969 were formula-, mixed-, and exclusively breastfed, respectively. Among girls, the mean APV was significantly later in the mixed-fed (standardized regression coefficient (β): 0.094, 95% confidence interval (CI): 0.004–0.180) and exclusively breastfed (β: 0.150, 95% CI: 0.056–0.250) groups than in the formula-fed group. Among boys, the mean APV was not significantly different among the three groups; however, a sensitivity analysis that excluded preterm birth revealed more significantly delayed APV in the breastfed-only group compared to the formula-fed group. Furthermore, a multiple linear regression model revealed that a longer breastfeeding period was associated with later APV.
Conclusions
Infant breastfeeding practices can affect the timing of peak height velocity in both boys and girls.
Impact
-
Several studies have discovered an association between infant feeding practices and puberty timing; however, most have involved female cohorts. Age at peak height velocity, derived from longitudinal height measurements, is a useful marker of secondary sexual maturity milestones in boys and girls.
-
A Japanese birth cohort study revealed that breastfed children had a later age at peak height velocity than their formula-fed counterparts; this was more prominent among girls than boys.
-
Furthermore, a duration-effect relationship was observed, where longer breastfeeding duration was associated with a later age at peak height velocity.
Similar content being viewed by others
Introduction
Puberty is the period of transition from childhood to adulthood and sexual maturity, during which bodily changes occur. The initial manifestations of secondary sexual characteristics are breast development in girls and increased testicular volume in boys. Another major change is a growth spurt, in which a marked acceleration in stature occurs during the early stages of puberty; approximately 20% of an adult’s height is gained during this period. The age at peak height velocity (APV) differs between sexes and is approximately 2 years and 1.5 years after puberty onset in boys and girls, respectively.
The timing and progression of puberty are affected by various factors, including race/ethnicity, genetics, psychosocial environment, endocrine-disrupting chemicals, and nutrition.1 Moreover, perinatal factors, such as maternal smoking, preterm birth, or small for gestational age (SGA) birth, may also predispose adolescents to early puberty onset.1,2,3,4,5 Several studies have discovered an association between infant feeding practices and puberty timing; however, most have involved female cohorts.6,7,8 A recent study revealed that girls who were not breastfed experienced an earlier onset of breast and pubic hair development than those who were breastfed for ≥6 months after adjusting for prepubertal body mass index (BMI).9 However, this association was insignificant among Asian/Pacific Islanders, possibly due to a smaller sample size, and it was not examined in boys.
The age of pubertal onset has decreased globally and has become a concern.10 A previous meta-analysis worldwide revealed that the age of breast development onset has decreased by 0.24 years per decade over the past 36 years.11 This trend toward earlier pubertal milestones can be partly attributed to the increasing prevalence of childhood obesity.12,13 In contrast, the timing of puberty onset and its association with obesity in boys remains controversial.12,14,15,16,17,18 This may be because secondary sexual characteristics in girls (such as breast enlargement or menarche) are easily recognized. In contrast, the onset of testicular enlargement is less conspicuous, and the cracking of the voice involves a relatively subjective evaluation, rendering the accurate estimation of the timing of puberty among boys more challenging.
We aimed to investigate the effect of infant breastfeeding practices on the timing of peak height velocity in boys and girls using data from a Nationwide Longitudinal Survey in Japan. We hypothesized that breastfed children would have a later peak height velocity onset at puberty than formula- or mixed-fed children.
Methods
Ethical considerations
All procedures in this study were performed in accordance with the 1964 Declaration of Helsinki and the 2003 Japanese Ethical Guidelines for Clinical Research and its subsequent amendments. This study was approved by the Institutional Review Boards of Okayama University Hospital (1506–073) and Okayama Medical Center (2019–027).
Participants
The Japanese Ministry of Health, Labour, and Welfare has conducted the “Longitudinal Survey of Newborns in the 21st Century” since 2001. This birth cohort investigation annually collected information on family circumstances, child-rearing, and children’s health and developmental statuses from all families in Japan to whom infants were born between January 10 and 17 and between July 10 and 17, 2001. The first set of questionnaires were mailed to 53,575 families when eligible infants reached the age of 6 months and were completed by 47,015 families (response rate, 88%). The form included questions regarding children’s perinatal status and household and socioeconomic factors such as residential area, maternal academic achievement level, and smoking status. Almost all (>98%) of the parents were Japanese, whereas most of the remainder were of Asian descent, reflecting the Japanese demographic composition at that time. Follow-up questionnaires including each child’s height and weight were sent at ages 1.5, 2.5, 3.5, 4.5, 5.5, 7, 8, 9, 10, 11, 12, 13, 14, and 15 years. Birth records from Japanese vital statistics, including birth weight, gestational age, single or multiple births, sex, parity, and maternal age at delivery, were retrieved and linked to the data. SGA, appropriate for gestational age (AGA), and large for gestational age (LGA) were defined as sex- and parity-specific birth weights below the 10th percentile, between the 10th and 90th percentiles, and above the 90th percentile for gestational age, respectively, based on the Japanese population reference. Maternal smoking status and educational attainment were obtained from the first and second surveys, respectively.
Infant feeding practices and anthropometric measurements
We obtained infant feeding methods and breastfeeding duration data from surveys at 6 months; the feeding methods were classified into three types: formula-fed (never breastfed other than colostrum, if applicable), mixed-fed (breastfeeding combined with formula), or exclusively breastfed. Children in the “exclusively breastfed” group were never formula-fed during the first 6–7 months of life. Heights and weights from the ages of 5.5 to 15 years were collected (the months these measurements were obtained). In Japan, children’s heights and weights are usually measured every semester for 15 years during the preschool and school years.
Data cleaning and analysis
Participants for whom data on all 10 height measurements (acquired over as many years), weight at 5.5 years, and infant feeding practice were available were included in the analysis. The data were cleaned to reduce the possibility of erroneous height values: height at age 5.5 years was defined as HT1, height at age 7 years as HT2, and so on until height at 15 years (HT10). Participants who did not satisfy the following formulae were excluded:
Boys: HT1 < HT2 < HT3 < HT4 < HT5 < HT6 < HT7 < HT8, HT8 < HT9 + 5, and HT9 < HT10 + 5
Girls: HT1 < HT2 < HT3 < HT4 < HT5 < HT6, HT6 < HT7 + 5, HT7 < HT8 + 5, HT8 < HT9 + 5, and HT9 < HT10 + 5
These formulae were set up to allow for monotonic increments in height, avoid duplication until late puberty stage, and remain within the maximum allowed measurement error or diurnal variation after reaching near-adult height.19 The thresholds for monotonic increment limits were set based on the Japanese population; −2 standard deviations ≤ APV ≤ first quartile had a near-adult height at approximately 14 years for boys and 12 years for girls.20
We used the SuperImposition by Translation and Rotation (SITAR) growth curve model to calculate the APV.21 SITAR is a non-linear multilevel model with natural cubic splines that estimates the population average growth curve and random effects; it captures differences in size, tempo, and velocity, thereby explaining an individual’s growth trajectory. The SITAR model allows flexibility in fitting spline curves by adjusting the degrees of freedom (for which we adopted 5 for both sexes).5 The variance score explained by the SITAR model was calculated. We obtained each participant’s estimated APV from the SITAR model with the “sitar” package (version 1.2.0) and the “iapvbs” package (version 0.0.2).22 The later package outputs the estimated APV with a flag value: 0 represents a normal estimated APV, 1 represents an estimated APV that is significantly close to the minimum or maximum age measurement, 2 represents an estimated APV equal to the minimum or maximum age measurement, 3 represents an estimated APV outside the range of age measurements, and 4 represents an estimated APV that is questionable owing to some other reasons.
Statistical analyses
Maternal and child characteristics and socioeconomic status were confounding factors based on the existing literature.3,4,5,6,7,8 Child factors included sex (dichotomous), birth weight (<2500, 2500–4000, and >4000 g, categorical), weight for gestational age (SGA, AGA, and LGA, categorical), term or preterm birth (<37 weeks’ gestation, dichotomous), and singleton or multiple births (dichotomous). Maternal factors included maternal age at delivery (<30, 30–34, and ≥35 years, categorical), smoking habits (nonsmoker, <10 daily cigarettes, and ≥10 daily cigarettes, categorical), maternal educational level (categorical), and residential area where the participant was born (ward, city, and town or village, categorical). We reclassified the original eight education categories into four: junior high school and others, high school, junior college (2 years) or vocational school, and university (4 years) or higher. In a previous study of the same population, almost all participants of both sexes who were overweight or obese at the age of 15 years had experienced adiposity rebound before 4.5 years; as such, we adopted the BMI at 5.5 years as the prepubertal BMI.23
Multiple linear regression was used to estimate the relationships after adjusting for the above confounders with and without prepubertal BMI, and the formula-fed group was used as a reference. To evaluate the association with preterm birth, we excluded participants with preterm births (n = 604) from the sensitivity analysis. In the additional linear regression analyses of the breastfeeding duration-effect relationship, we subcategorized infant feeding types into exclusively formula-fed, formula-fed plus only colostrum, breastfed (combined with formula) for 1–2 months, 3–5 months, and 6–7 months, and exclusively breastfed at 6–7 months. The main analysis was performed using the R statistical software (R Foundation, Vienna, Austria, version 4.1.2) through R Studio (RStudio, Boston, America, 2021.09.1 + 372 “Ghost Orchid”). The statistical analyses were performed using Stata 17 (StataCorp). A p < 0.05 was considered statistically significant.
Results
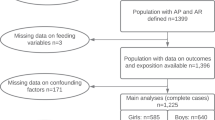
Among the Longitudinal Survey of Newborns in the 21st Century cohort, 15,998 (29.9%) participants had complete anthropometric measurement data available for the analysis period. The data-cleaning process reduced the number of participants to 13,162; the variance scores attained by the aforementioned model increased from 89.59 to 92.93 for boys and 89.55 to 93.08 for girls. After excluding participants lacking data on feeding practice history, we finally included 13,074 participants (Fig. 1), 98.7% of whom were Japanese parents. The baseline characteristics of eligible and ineligible participants were similar, except for fewer maternal smoking habits and higher maternal educational attainment among the eligible population (Table 1).
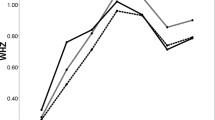
Among the 13,074 participants, 650 (5.0%) were formula-fed, 9455 (72.3%) were mixed-fed, and 2969 (22.7%) were exclusively breastfed at 6–7 months of age. Twenty-six boys and one girl were identified as Flag 1, 103 boys as Flag 2, and none as Flag 3 or 4. The demographic characteristics of the participants and their APVs, sorted according to infant feeding practices, are presented in Table 2. The APVs distribution chart in boys and girls are shown in Supplementary Fig. 1.
The results of multiple linear regression analyses of infant feeding practices and APV, adjusted for child- and maternal-related factors, are presented in Table 3. After adjusting for covariates, mixed feeding and breastfeeding were associated with later APV among the girls. Additionally, a higher prepubertal BMI was negatively correlated with the APV in boys (standardized regression coefficient (β): −0.099, p < 0.001, 95% confidence interval (CI): −0.11 to −0.083) and girls (β: −0.15, p < 0.001, 95% CI: −0.16 to −0.13). Excluding prepubertal BMI analysis was also consistent with the result (mixed-fed [β: 0.11, 95% CI: 0.039–0.180] and exclusively breastfed groups [β: 0.15, 95% CI: 0.081–0.230] had higher values compared with the formula-fed group [both sexes combined]). Our analysis also revealed that SGA birth, smoking ≥10 cigarettes per day, and maternal age ≥25 years at delivery were positively associated with early APV among boys; however, only SGA birth was significantly associated with early APV among girls. Sensitivity analysis, which excluded preterm infants, revealed that mixed-fed and breastfed infants had a later APV than formula-fed infants, including boys (Table 4).
Furthermore, linear regression analysis using breastfeeding duration as the exposure revealed a duration-effect relationship, with a later APV associated with a longer breastfeeding duration (Table 5). In addition, sub-analysis (formula-fed with/without colostrum group as a reference) revealed similar results (Supplementary Table 1). The variance inflation factors for Tables 3, 5, and Supplementary Table 1 are shown in Supplementary Fig. 2.
Discussion
In this nationwide birth cohort study, we observed that infant breastfeeding practices affected the timing of peak height velocity. Breastfed children had later APVs than their formula-fed counterparts; this was more pronounced among girls than boys. In the sensitivity analysis, excluding preterm birth data strengthened these associations; they became significant among boys. Furthermore, multiple regression analysis revealed that longer breastfeeding duration was associated with later APV.
It is important to consider racial disparity in pubertal development. A prospective cohort study from the United States discovered that thelarche onset for girls who were exclusively formula-fed was approximately 2.5 months earlier than in those who were breastfed for more than 6 months. However, the association was only evident among African Americans.9 Another study in the United States that revealed similar results included a small number of Asians (57 out of 1237 study participants; 15 were predominantly breastfed, 33 were mixed-fed, and six were formula-fed).8 An observational study from Korea discovered that early puberty occurred less frequently in children who were breastfed for 6 months or longer than in those who were not (odds ratio: 0.37; 95% CI: 0.18–0.74); however, the participants were not stratified by sex.24 A study of Chinese children conducted in Hong Kong discovered that breastfeeding was not associated with pubertal timing. However, this study defined breastfed infants as those breastfed for at least 3 months.25 Our cohort, composed almost entirely of Japanese individuals, revealed a significant difference in APV among those breastfed for 6 months or longer. As exclusive breastfeeding is recommended by the World Health Organization and United Nations Children’s Fund for the first 6 months of life, defining breastfeeding using the interval endorsed by these agencies should be more appropriate.26,27 Furthermore, a longer and more frequent mother-infant skin-to-skin contact may affect the APV; however, no information was collected on breastfeeding style (direct breastfeeding or bottle-feeding pumped breastmilk).28,29 Some studies that support the beneficial outcomes of breastfeeding may be subject to publication bias; 30however, our findings were consistent with those of other investigators.
In our previous analysis of the same population, exclusive breastfeeding at 6–7 months was associated with a lower risk of overweight/obesity at 7 years than formula feeding.31 Another recent study revealed that early adiposity rebound was associated with overweight/obesity at 15 years and that the APV was earlier in the overweight/obese groups than in the normal-weight group.23 Our current study expanded on the associations between infant feeding practices and pubertal timing, particularly after considering potential confounding factors, such as prepubertal BMI and perinatal circumstances. A recent prospective birth cohort study of predominantly Black mothers and children using the SITAR model also revealed that obesity at 5–7 years was associated with an earlier APV in boys, whereas overweight/obesity at 5–7 years was associated with an earlier APV in girls.32 The association between infant feeding practices and puberty has been confounded by obesity, especially in girls. This study revealed an exposure-response relationship between breastfeeding duration and APV, regardless of sex, after adjusting for prepubertal BMI, suggesting a direct effect of infant feeding practices on puberty.
A recent large cohort study investigated the association between breastfeeding and pubertal signs stratified by sex.33 The shorter duration of exclusive breastfeeding was significantly associated with earlier pubertal development in boys but not girls. Our findings were the opposite in boys and girls. Some covariates also revealed significant differences between boys and girls; however, the reason for this is unknown. SGA children tend to experience early puberty than AGA counterparts owing to catch-up growth with rapid weight gain in early childhood; this is believed to cause increased visceral adiposity, decreased insulin sensitivity, and elevated insulin-like growth factor-I levels.34 Our data also revealed that SGA birth was associated with earlier APVs than AGA birth. One study published in 1995 suggested an association between preterm birth and precocious puberty; however, no such association was observed in a later systematic review.3 A recent study of a Finnish birth cohort that used the SITAR model also revealed no association.5 In our sensitivity analysis, wherein preterm infants were excluded, the association between formula feeding and early puberty was reaffirmed or even stronger. A Danish national birth cohort study revealed an association between maternal prenatal smoking and early puberty.4 Our analysis used information on environmental smoking exposure in infants and revealed similar results only among boys. The overall mechanism for puberty onset has not been elucidated; however, various exposures from the prenatal period to infancy can affect puberty timing, which manifests even more than 10 years later.
APV, derived from longitudinal measurements of height, is a useful marker of secondary sexual maturity milestones, such as Tanner staging or voice cracking. Frequent and precise assessment of secondary sexual characteristics is challenging, especially in a large population of children. Some studies relied on self-assessment for Tanner staging; however, objectively assessing the progression of secondary sexual development other than menarche may have been overlooked.28,35,36 A birth cohort study in the United Kingdom examined breastfeeding duration and puberty timing after adjusting for depression during pregnancy.37 Mothers with higher anxiety levels were less likely to breastfeed, and longer breastfeeding duration was associated with later menarche; however, no association was discovered between breastfeeding and voice change in males. APV is an objective measure less susceptible to subjective and recall biases.
This study had some limitations. Its longitudinal nature makes it susceptible to missing unmeasured confounders for breastfeeding and health outcomes. Parental information, such as maternal pre-pregnancy BMI and gestational diabetes mellitus, and genetic factors, such as age at maternal menarche and paternal APV, were unavailable. Prepubertal BMI can reflect genetic factors from parents and the rearing environment as confounders and an intermediate factor of exposure to breastfeeding. This significance was attenuated when the prepubertal BMI was included as a covariate. Additionally, we adjusted for maternal characteristics (smoking status and educational level); however, we could not exclude the possibility of other residual confounding factors. Moreover, we did not obtain data on the children’s adiposity or body composition measurements, which are also associated with pubertal timing.38 We could not rule out the possibility that children in the exclusively breastfed group could have received liquid (water and sugar beverages) or complementary food in the first survey; thus, the exclusively breastfed group might have included some children not complying with the World Health Organization and United Nations Children’s Fund recommendation strictly. Almost all study participants were Japanese, and generalizability to other populations may be limited. The reliability of anthropometric data and the validity of the data cleaning process also remain concerns. Height and weight measurements were reported by parents, which may be less reliable than obtaining these measurements from official healthcare records. Moreover, the number of children included in the analysis was constrained by the large amount of missing anthropometric data. The SITAR model can be used with a few measurements; however, we excluded participants with missing values to obtain a more accurate estimate. Additionally, data cleaning to exclude participants up to a certain age whose height decreased relative to the preceding year increased the explanatory score of the model; however, more “rigorous” cleaning, which may have increased its value even further, was not performed. No universally agreed standard methods for cleaning height measurement data currently exist.39
In conclusion, our data demonstrate that infant breastfeeding practices affect the timing of peak height velocity in both boys and girls. Moreover, we observed a duration-effect relationship, wherein a later APV was observed in participants who experienced a longer breastfeeding period after adjusting for perinatal and socioeconomic confounding factors.
Data availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under licenses. The corresponding author will accept requests to access the data and provide details regarding the restrictions and conditions under which such access may be provided.
References
Duan, R. et al. The overall diet quality in childhood is prospectively associated with the timing of puberty. Eur. J. Nutr. 60, 2423–2434 (2021).
Chen, Y. et al. Association of prenatal and childhood environment smoking exposure with puberty timing: a systematic review and meta-analysis. Environ. Health Prev. Med. 23, 1–2 (2018).
James, E., Wood, C. L., Nair, H. & Williams, T. C. Preterm birth and the timing of puberty: a systematic review. BMC Pediatr. 18, 1–2 (2018).
Brix, N. et al. Maternal smoking during pregnancy and timing of puberty in sons and daughters: a population-based cohort study. Am. J. Epidemiol. 188, 47–56 (2019).
Suikkanen, J. et al. Preterm birth and subsequent timing of pubertal growth, menarche, and voice break. Pediatr. Res. 92, 199–205 (2022).
Morris, D. H. et al. Determinants of age at menarche in the UK: analyses from the breakthrough generations study. Br. J. Cancer 103, 1760–1764 (2010).
Al-Sahab, B. et al. Impact of breastfeeding duration on age at menarche. Am. J. Epidemiol. 173, 971–977 (2011).
Kale, A. et al. Breastfeeding versus formula-feeding and girls’ pubertal development. Matern Child Health J. 19, 519–527 (2015).
Aghaee, S. et al. Breastfeeding and timing of pubertal onset in girls: a multiethnic population-based prospective cohort study. BMC Pediatr. 19, 277 (2019).
Biro, F. M. et al. Onset of breast development in a longitudinal cohort. Pediatrics 132, 1019–1027 (2013).
Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. 174, e195881 (2020).
Brix, N. et al. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J. Epidemiol. 49, 834–844 (2020).
Ferrari, V. et al. Retrospective longitudinal analysis of the effects of postnatal weight gain on the timing and tempo of puberty and menarche in a cohort of Italian girls. Ital. J. Pediatr. 48, 1–6 (2022).
Lee, J. M. et al. Timing of puberty in overweight versus obese boys. Pediatrics 137, e20150164 (2016).
Aydin, B. K. et al. The relationship between infancy growth rate and the onset of puberty in both genders. Pediatr. Res 82, 940–946 (2017).
Bygdell, M. et al. Childhood BMI is inversely associated with pubertal timing in normal-weight but not overweight boys. Am. J. Clin. Nutr. 108, 1259–1263 (2018).
Ohlsson, C. et al. Secular trends in pubertal growth acceleration in Swedish boys born from 1947 to 1996. JAMA Pediatr. 173(9), 860–865 (2019).
Busch, A. S., Højgaard, B., Hagen, C. P. & Teilmann, G. Obesity is associated with earlier pubertal onset in boys. J. Clin. Endocrinol. Metab. 105, E1667–E1672. (2020).
Siklar, Z., Sanli, E., Dallar, Y. & Tanyer, G. Diurnal variation of height in children. Pediatr. Int. 47, 645–648 (2005).
Yoshii, K. & Tanaka, T. Establishment of a longitudinal growth chart corresponding to pubertal timing. Clin. Pediatr. Endocrinol. 27, 215–224 (2018).
Cole, T. J., Donaldson, M. D. C. & Ben-shlomo, Y. SITAR-a useful instrument for growth curve analysis. Int J. Epidemiol. 39, 1558–1566 (2010).
Cao, Z., Hui, L. L. & Wong, M. Y. New approaches to obtaining individual peak height velocity and age at peak height velocity from the SITAR model. Comput Methods Prog. Biomed. 163, 79–85 (2018).
Matsumoto, N. et al. Trajectory of body mass index and height changes from childhood to adolescence: a nationwide birth cohort in Japan. Sci. Rep. 11, 23004 (2021).
Lee, H. A. et al. The preventive effect of breast-feeding for longer than 6 months on early pubertal development among children aged 7–9 years in Korea. Public Health Nutr. 18, 3300–3307 (2015).
Kwok, M. K., Leung, G. M., Lam, T. H. & Schooling, C. M. Breastfeeding, childhood milk consumption, and onset of puberty. Pediatrics 130, e631–e639 (2012).
Global strategy for infant and young child feeding The optimal duration of exclusive breastfeeding. Available at: https://apps.who.int/iris/bitstream/handle/10665/78801/ea54id4.pdf.
Infant and young child feeding. Available at: https://www.who.int/en/news-room/fact-sheets/detail/infant-and-young-child-feeding.
DiSantis, K. I., Collins, B. N., Fisher, J. O. & Davey, A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle?. Int J. Behav. Nutr. Phys. Act. 8, 89 (2011).
Pang, W. W. et al. Nutrients or nursing? Understanding how breast milk feeding affects child cognition. Eur. J. Nutr. 59, 609–619 (2020).
Villamor, E. & Jansen, E. C. Nutritional determinants of the timing of puberty. Annu Rev. Public Health 37, 33–46 (2016).
Yamakawa, M. et al. Breastfeeding and obesity among schoolchildren: a nationwide longitudinal survey in Japan. JAMA Pediatr. 167, 919–925 (2013).
Chen, L. K. et al. Trajectory of body mass index from ages 2 to 7 years and age at peak height velocity in boys and girls. J. Pediatr. 230, 221–229.e5 (2021).
Hvidt, J. J. et al. Breast feeding and timing of puberty in boys and girls: a nationwide cohort study. Paediatr. Perinat. Epidemiol. 35, 578–589 (2021).
Netchine, I. et al. New horizons in short children born small for gestational age. Front Pediatr. 9, 655931 (2021).
Brix, N. et al. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J. Epidemiol. 49, 834–844 (2021).
Diao, H., Wang, H., Yang, L. & Li, T. The association between sleep duration, bedtimes, and early pubertal timing among Chinese adolescents: a cross-sectional study. Environ. Health Prev. Med. 25, 1–8 (2020).
English, S. et al. Prenatal anxiety, breastfeeding and child growth and puberty: linking evolutionary models with human cohort studies. Ann. Hum. Biol. 47, 106–115 (2020).
Li, Y. et al. Adiposity status, trajectories, and earlier puberty onset: results from a longitudinal cohort study. J. Clin. Endocrinol. Metab. 107, 2462–2472 (2022).
Woolley, C. S. C. et al. Is it time to stop sweeping data cleaning under the carpet? A novel algorithm for outlier management in growth data. PLoS One 15, e0228154 (2020).
Acknowledgements
We thank Elsevier Language Editing services (https://webshop.elsevier.com/language-editing-services/language-editing/) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.H. conceived of and designed the study. T.Y. contributed substantially to the data acquisition. Y.H. and N.M. performed data analysis. S.F., Y.E., M.F., K.N., and T.K. contributed to interpretation of data. Y.H. drafted the manuscript, and all other authors reviewed and revised the manuscript critically for important intellectual content. All the authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Higuchi, Y., Matsumoto, N., Fujiwara, S. et al. Association between infant breastfeeding practices and timing of peak height velocity: A nationwide longitudinal survey in Japan. Pediatr Res 94, 1845–1854 (2023). https://doi.org/10.1038/s41390-023-02706-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02706-y