Abstract
Background
We aimed to investigate the effect of epinephrine vs placebo on return of spontaneous circulation (ROSC) and brain magnetic resonance spectroscopy and imaging (MRS/MRI) in newborn piglets with hypoxic cardiac arrest (CA).
Methods
Twenty-five piglets underwent hypoxia induced by endotracheal tube clamping until CA. The animals were randomized to CPR + intravenous epinephrine or CPR + placebo (normal saline). The primary outcome was ROSC, and secondary outcomes included time-to-ROSC, brain MRS/MRI, and composite endpoint of death or severe brain MRS/MRI abnormality.
Results
ROSC was more frequent in animals treated with epinephrine than placebo; 10/13 vs 4/12, RR = 2.31 (95% CI: 1.09–5.77). We found no difference in time-to-ROSC (120 (113–211) vs 153 (116–503) seconds, p = 0.7) or 6-h survival (7/13 vs 3/12, p = 0.2). Among survivors, there was no difference between groups in brain MRS/MRI. We found no difference in the composite endpoint of death or severe brain MRS/MRI abnormality; RR = 0.7 (95% CI: 0.37–1.19).
Conclusions
Resuscitation with epinephrine compared to placebo improved ROSC frequency after hypoxic CA in newborn piglets. We found no difference in time-to-ROSC or the composite endpoint of death or severe brain MRS/MRI abnormality.
Impact
-
In a newborn piglet model of hypoxic cardiac arrest, resuscitation with epinephrine compared to placebo improved the rate of return of spontaneous circulation and more than doubled the 6-h survival.
-
Brain MRS/MRI biomarkers were used to evaluate the effect of epinephrine vs placebo. We found no difference between groups in the composite endpoint of death or severe brain MRS/MRI abnormality.
-
This study adds to the limited evidence regarding the effect and safety of epinephrine; the lack of high-quality evidence from randomized clinical trials was highlighted in the latest ILCOR 2020 guidelines, and newborn animal studies were specifically requested.
Similar content being viewed by others
Introduction
Epinephrine is an integral component of neonatal resuscitation guidelines.1,2,3,4,5 However, the effect and safety of epinephrine remains controversial. In animals, epinephrine-mediated peripheral vasoconstriction increases coronary perfusion pressure (CPP) and ultimately the chance of achieving return of spontaneous circulation (ROSC).6,7,8 However, animal studies also suggest that epinephrine may compromise cerebral microvascular blood flow and myocardial function.9,10 Similar concerns exist in adult cardiac arrest (CA).11 The association between advanced cardiopulmonary resuscitation (CPR), i.e., including chest compressions and epinephrine, and poor neurodevelopmental outcome is well known,12,13,14 and epinephrine may improve short-term survival but at the cost of poorer long-term outcomes.11
Magnetic resonance spectroscopy and imaging (MRS/MRI) are used as prognostic tools to predict neurological impairment in newborns suffering from encephalopathy after a hypoxic–ischemic insult.15 Lactate/N-acetyl-aspartate ratio (Lac/NAA) measured in the thalamus (Thal) by MRS is considered one of the most accurate early biomarkers for prediction of neurologic outcome.15,16 High Lac/NAA ratio (>0.39) measured in deep gray matter in infants with hypoxic ischemic encephalopathy (HIE) treated with therapeutic hypothermia (TH) has been associated with adverse or fatal neurologic outcome.17,18
The aim of this randomized controlled study was to evaluate the effect of epinephrine in a piglet model of neonatal hypoxic CA. Our primary outcome was ROSC, and the secondary outcomes were time-to-ROSC, 6-h brain MRS/MRI, and composite endpoint of death or severe brain MRS/MRI abnormality.
Materials and methods
Ethics
The study was approved by The Danish Animal Experiments Inspectorate (2018-15-0201-01555). The experiments were conducted in 2019. Reporting complies with the ARRIVE 2.0 guidelines (Supplementary S1).19
Study design
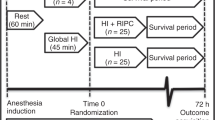
The study was a randomized placebo-controlled study. Two animals from the same litter and of same sex were included at a time. Prior to CA induction, one animal was randomized to either epinephrine (0.01 mg/kg, equating a volume of 0.1 ml/kg) or placebo (0.1 ml/kg 0.9% saline), and the other animal received the treatment different from the randomized littermate. All investigators were blinded to the allocated treatment during the entire study. The corresponding author was in charge of the study medications; each day, the syringes were filled with an equal volume of epinephrine or placebo. An independent person, not participating in the experiments, labeled the syringes with “Heads” or “Tails”, and logged the details in a document inaccessible to the researchers. We used the online randomization tool “Just flip a coin.”20 After randomization, the volume of the syringes was adjusted to the weight of the animal.
Animal preparation
We included Danish Landrace piglets of either sex, under 12 h of age, and weight between 1500 and 2000 g. Anesthesia was induced by inhalation of 2–4% sevoflurane. Peripheral venous access was obtained and a bolus of propofol (10 mg/kg), fentanyl (30 μg/kg), and rocuronium (1 mg/kg) was administered. Animals were intubated orally and mechanically ventilated, adjusted to maintain an end-tidal CO2 of 4.5–5.5 kPa. Anesthesia was maintained by continuous infusion of propofol (4–12 mg/kg/h) and fentanyl (5–10 μg/kg/h). Anesthesia was paused during CA and resuscitation and continued after ROSC at the lowest relevant dose. A 3.5 Fr catheter was inserted in an umbilical artery for blood sampling and continuous measurement of mean arterial blood pressure (MABP) and a 3.5 or 5.0 Fr catheter was inserted in the umbilical vein for fluid and drug administration. Prophylactic intravenous gentamicin 5 mg/kg and ampicillin 30 mg/kg were administered prior to placement of umbilical catheters. Normal values of blood glucose and normohydration were maintained by continuous intravenous infusion of 5–10 mL/kg/h NeoKNaG (Na+ 15 mmol/L, K+ 10 mmol/L, Cl− 25 mmol/L, glucose 505 mmol/L). Core temperature was maintained at 38.5–39 °C (normothermia for piglets) by a heated inflatable air mattress. Percutaneous oxygen saturation, electrocardiography, MABP, and heart rate (HR) were measured continuously. Arterial blood samples were analyzed (ABL Radiometer Medical, Denmark) to monitor blood electrolytes, CO2, O2, glucose, pH, and lactate.
CA and resuscitation protocol
Baseline information was obtained 30 min prior to CA induction. CA was induced by clamping the endotracheal tube (ETT). CA was defined as MABP < 20 mm Hg and HR <60 bpm (assessed by auscultation or arterial pressure curve). CPR was initiated 5 min after the blood pressure and HR criteria were obtained to ensure a period of no flow (HR = 0 and MABP = 0). CPR was performed according to ILCOR 2015 guidelines.1 Potential ROSC was evaluated every 30 s. ROSC was defined as MABP > 40 mm Hg or HR > 100 bpm sustained for 5 min. Time-to-ROSC was registered at the beginning of the 5 min. Resuscitation was commenced by restoring ventilation (FiO2 = 21%, rate = 40/min, positive end expiratory pressure = 5 cm H2O, and peak inspiratory pressure = 15 cm H2O). If CA persisted after 30 s of ventilation, manual chest compressions were initiated asynchronous to ventilations at a rate of 3:1 (90 compressions and 30 ventilations per minute), a depth of 1/3 of the thoracic anteroposterior diameter, using the two-thumb encircling technique. Simultaneously, FiO2 was adjusted to 100%. If ROSC was not achieved after 30 s of chest compression, epinephrine or placebo was administered through the umbilical venous catheter. If CA persisted, administration was repeated every 4 min1 for a maximum of six doses. Resuscitative efforts were discontinued if ROSC was not achieved within 30 min.
If successfully resuscitated, the animals were treated with TH, initiated by placing 5 °C water bags directly on the piglet. Target core temperature was between 33 and 34 °C. Anesthetic and hemodynamic support was titrated according to protocol (Supplementary S2). The duration of post-resuscitation observation and monitoring was 6 h. Euthanasia occurred under general anesthesia via injection of a lethal dose of pentobarbital (80 mg/kg).
Magnetic resonance spectroscopy and imaging
Six hours post ROSC, MRS/MRI was performed using a 3.0 T system (Skyra model; Siemens, Germany), according to a standard protocol, previously established by our group21 (Supplementary S3). Briefly, proton MRS (repetition-time/echo-time: 2000/135 ms) was obtained from 8 × 8 × 8 mm3 voxels. Lac/NAA and NAA/creatine (NAA/Cr) ratio were assessed in frontal cortex (fCTX), occipital cortex (oCTX), Thal, and white matter (WM). Diffusion-weighted images (DWI) were attained, and the apparent diffusion coefficient value (ADC) was calculated. Blood-oxygenation level-dependent (BOLD) imaging was employed using a multi-echo gradient-echo sequence, and the apparent relaxation constant T2* was calculated. MRS/MRI data analyses were blinded to treatment allocation.
Composite endpoint analysis of death or severe brain MRS/MRI abnormality
Severe brain MRS/MRI abnormality was defined as values within the upper quartile of thalamic Lac/NAA ratio.15
Statistics
The scarcity of previously published studies prevented an informed power calculation. Accounting for the potential number of animals achieving ROSC before treatment intervention, mortality, and animal ethics, we estimated that 14 animals per group were sufficient for analysis.
Statistical analyses were performed using GraphPad PRISM version 8.00 (GraphPad Software, San Diego, CA). Dichotomous data (ROSC, death post ROSC, and vasopressor treatment) were analyzed by Fisher’s exact test and reported as relative risk (RR) with 95% confidence interval (CI). Continuous variables (time-to-CA, time-to-ROSC, Lac/NAA ratio, NAA/Cr ratio, ADC, BOLD, and hemodynamic and metabolic variables) were compared by Student’s t test (if normally distributed) and presented as mean values with standard deviation (±SD) or by Mann–Whitney rank-sum test (if non-normally distributed) and presented as median values with interquartile range. The composite endpoint of death or severe brain MRS/MRI abnormality was reported as RR with 95% CI. A sensitivity analysis including animals with missing MRS/MRI data was conducted. Hemodynamic and metabolic variables were graphed based on data from mixed-effects analyses, with assumed sphericity, missing values at random, and corrected for multiple comparison by Bonferroni’s test. A two-sided p value <0.05 was considered statistically significant.
Results
One of the 28 animals was not randomized due to unsuccessful arterial access. ROSC occurred before treatment intervention in 2 of the remaining 27 animals (one from each group); these animals were euthanized after ROSC. All 27 animals were included in an intention to treat analysis (Supplementary S4). The two animals that achieved ROSC before treatment were excluded from the final analysis as described in ref. 22. The following results are based on per protocol analysis of 25 animals: epinephrine (n = 13) vs placebo (n = 12). We found no difference in results analyzed according to intention to treat vs per protocol. A flow chart is presented in Supplementary S5.
Baseline and CA characteristics
Baseline characteristics and characteristics at the time of CA are shown in Table 1. Hemodynamic and metabolic variables from baseline to the end of experiment are shown in Fig. 1. Blood glucose at baseline was higher in the epinephrine group compared to the placebo group. This difference was not observed at CA or after ROSC. In all animals, the primary arrest rhythms were asystole or pulseless electrical activity. Conversion from the primary non-shockable rhythm to ventricular fibrillation (VF) was observed in 7/13 (54%) animals in the epinephrine group and 4/12 (33%) in the placebo group, RR = 1.6 (95% CI: 0.7–4.3). Rhythm conversion occurred after administration of epinephrine or placebo.
a Rectal temperature, b heart rate (HR), c mean arterial blood pressure (MABP), d pH, and e lactate. Number of animals alive at each timepoint is denoted below the x-axis, color denotes group. Number of animals per group; epinephrine/placebo: BL (n = 13/12), CA (n = 13/12), 1 h (n = 10/4), 2 h (n = 10/4), 4 h (n = 9/4), 6 h (n = 7/3). Rectal temperature, HR, and pH are means ± standard deviation. MABP and lactate are medians with interquartile range.
Intra arrest and early post ROSC hemodynamics
During minutes 2–10 after resuscitation onset, animals treated with epinephrine had significantly higher aortic systolic blood pressure (SBP) (96.0 ± 8.5 vs 55.2 ± 5.5 mm Hg, p < 0.0001), aortic diastolic blood pressure (DBP) (54.1 ± 4.5 vs 24.8 ± 1.6 mm Hg, p < 0.0001), and MABP (68.8 ± 5.8 vs 33.9 ± 2.5 mm Hg, p < 0.0001) compared to animals treated with placebo (values are means from the entire 8-min period). Correspondingly, in animals that achieved ROSC, SBP (121.2 ± 10.7 vs 87.4 ± 8.9, p < 0.0001), DBP (74.5 ± 7.2 vs 53.1 ± 7.2, p < 0.0001), and MABP (92.9 ± 8.9 vs 67.7 ± 8.1, p < 0.001) were significantly higher in animals resuscitated with epinephrine compared to placebo during minutes 2–10 after resuscitation onset (values are means from the entire 8-min period) (Fig. 2).
a Systolic blood pressure, b diastolic blood pressure, and c mean arterial blood pressure. The first timepoint represents 30 s of ventilation. BP blood pressure, MABP mean arterial blood pressure. Values are means ± standard deviation. Compared by mixed-effects analyses and corrected for multiple comparison by Bonferroni’s test. *p < 0.05 epinephrine vs placebo. **p < 0.01 epinephrine vs placebo. ***p < 0.001 epinephrine vs placebo. ****p < 0.0001 epinephrine vs placebo. Remaining group comparisons (a–c): Epinephrine–ROSC vs epinephrine–no ROSC: p < 0.05 from 90 s and forward. Epinephrine–ROSC vs placebo–no ROSC: p < 0.05 from 90 s and forward. Epinephrine–no ROSC vs placebo –no ROSC: NS at all timepoints, epinephrine–no ROSC vs placebo–ROSC: p < 0.05 from 90 s and forward. Placebo–ROSC vs placebo–no ROSC: p < 0.05 from 90 s and forward.
The majority of the animals (10/14) achieved ROSC within the first 3 min of resuscitation. During minutes 2 and 3 of active resuscitation, all blood pressure values were higher in animals that received epinephrine compared to placebo (Table 2).
ROSC and 6-h survival
Animals that received epinephrine had significantly higher rate of ROSC (epinephrine 10/13 vs placebo 4/12, RR 2.31; 95% CI: 1.09 to 5.77, p = 0.047). We found no difference between the groups in median time-to-ROSC (epinephrine: 120 (113–211) vs placebo 153 (116–503) s, p = 0.66), and no difference in 6-h survival (7/13 vs 3/12, p = 0.23).
Due to refractory hypotension, 4 of the 14 animals in which ROSC was achieved died before the 6-h MRS/MRI examination (epinephrine: 3/10 vs placebo: 1/4, RR = 0.9 (95% CI: 0.5–2.5)). One additional animal was euthanized prior to MRS/MRI due to technical issues. Thus, nine animals completed MRS/MRI (epinephrine: n = 6 vs placebo: n = 3) (Supplementary S4). We found no difference in post-ROSC norepinephrine use between groups (epinephrine: n = 8/10, median infusion rate: 0.25 μg/kg/min (0.13–0.63) vs placebo: n = 4/4, median infusion rate: 0.4 μg/kg/min (0.27–0.97), p = 0.23). One piglet (randomized to epinephrine) received hydrocortisone and did not survive to MRS/MRI.
Magnetic resonance spectroscopy
There was no difference between animals that received epinephrine and placebo in Lac/NAA ratio measured in fCTX, oCTX, and Thal (Fig. 3). In WM, we were unable to detect lactate peaks in 3/6 animals resuscitated with epinephrine and 3/3 animals resuscitated with placebo. We found no difference between groups in NAA/Cr ratio measured in fCTX, oCTX, Thal, or WM (Fig. 3).
In total, 1/6 animals resuscitated with epinephrine and 1/3 animals resuscitated with placebo had severe MRS/MRI abnormality.
Magnetic resonance imaging
Cerebral edema quantified by DWI was similar in animals resuscitated with epinephrine vs placebo (Fig. 4). MRI-BOLD showed no difference in cerebral oxygenation between the two groups (Fig. 4).
Composite endpoint of death or severe brain MRS/MRI abnormality
We found no difference in the composite endpoint of death or severe brain MRS/MRI abnormality between animals resuscitated with epinephrine compared to placebo, RR = 0.7 (95% CI: 0.37–1.19). When including the only animal with missing MRS/MRI data in a sensitivity analysis assigning the piglet to a good or severe MRS/MRI outcome, the RR was 0.65 (95% CI: 0.34–1.12) and 0.74 (95% CI: 0.41–1.23), respectively.
Discussion
The use of epinephrine compared to placebo during resuscitation in piglets with hypoxic CA resulted in more than twice as many piglets with ROSC but no difference in the composite endpoint of death or severe brain MRS/MRI abnormality.
Our findings related to ROSC are consistent with some, but not all, previous experimental animal studies. Sobotka et al.23 reported that chest compressions alone were insufficient to achieve ROSC and that epinephrine was critical to increase HR, carotid arterial pressure, and cerebral blood flow. Mendler et al.24 and Solevåg et al.25 also found that at least one dose of epinephrine was required for successful resuscitation. In contrast, Wagner et al.,26 Linner et al.,27 and McNamara et al.28 concluded that epinephrine failed to increase the rate of ROSC. These conflicting results are likely explained by differences in the study protocols. The most striking difference between the three former studies and the three later studies was the CA criteria. Wagner et al.26 defined CA as HR < 25% of baseline, and Linner et al.27 defined CA as HR < 50 bpm and MABP < 25 mm Hg; however, if the criteria were unmet for 12 min of apnea, resuscitation was commenced. Accordingly, the hypoxic insult may have been less severe in these studies. In the study by McNamara et al.,28 the CA criteria were similar to ours, while the no-flow period was only 4 min compared to 5 min in our study. Further, epinephrine was administered 3 min after resuscitation onset, compared to 1 min in our study. Studies of pediatric CA have shown that the chance of achieving ROSC is decreased by every minute epinephrine administration is delayed.29,30 Additionally, McNamara et al.28 only administered one dose of epinephrine and resuscitation was discontinued if ROSC was not achieved within 6 min after chest compression onset.
We used a flush volume of 1 ml after epinephrine.31 However, there is concern that this volume may be inadequate to deliver the epinephrine into the right atrium and the systemic circulation during resuscitation. In a recent preclinical study, a flush volume of 2.5 mL increased the chance of achieving ROSC compared to a flush volume of 1 mL.32 Our rate of ROSC in animals resuscitated with epinephrine compared to placebo may therefore increase even more with a flush volume of 2.5 mL.
Newborn animal models of CA have failed to produce convincing evidence to support the use of epinephrine in neonatal resuscitation. This may be explained by differences in the CA models used. Our rationale behind introducing a new CA model was to establish a highly translatable and reproducible model of hypoxic CA with relevant insult severity. We find ETT clamping to be a more clinically relevant approach compared to existing approaches like “normocapnic hypoxia,”26,33,34 “ventricular fibrillation,”35,36 or “progressive hypoxia with added CO2.”24,37 Normocapnia is not common during clinical perinatal hypoxia and ischemia, and ventricular fibrillation is rarely the primary arrest rhythm. ETT clamping produces relevant hypoxemia, hypercapnia, and mixed respiratory and metabolic acidosis, which replicates the biochemistry in newborn hypoxic–ischemic CA. The CA criteria presented in this study produced a hypoxic–ischemic insult of relevant severity that ensured few animals with ROSC before drug administration without an excessive mortality; only 2 of the 28 piglets achieved ROSC before drug administration, which is few compared to other neonatal CA models.26,28,34
Conversion from a non-shockable rhythm to a shockable rhythm (treated) is a good prognostic sign in pediatric CA.38 As per the current standard for human neonatal resuscitation, defibrillation was not part of our procedure, and we observed no difference in the rate of ROSC between animals with and without VF in either experimental group (data not shown). Swine are known to be more arrhythmogenic than humans.39 High frequencies of VF were also observed in ref. 28 This emphasizes the necessity of studies of arrest rhythms in human neonates.
Animal studies have established that adequate myocardial perfusion is an essential determinant of ROSC after CA.40,41,42 Myocardial perfusion is primarily driven by CPP; however, DBP has proven a relevant surrogate measure of CPP.43 Substantial information from more mature swine models has established the benefit of epinephrine on hemodynamics, which is associated with ROSC, survival post ROSC, and survival with favorable neurologic outcome.7,40,41 In a swine model of pediatric CA, DBP > 26 mm Hg after first dose of epinephrine was associated with 95% survival (survival = sustained ROSC for 20 min).44 A recent clinical study demonstrated that DBP > 25 mm Hg in term newborns and >30 mm Hg in children (>1 year of age) improved survival and survival with favorable neurologic outcome after CA.45 Our study was not designed to identify the optimal DBP value during neonatal resuscitation; however, our results support these previous findings. We observed higher rates of ROSC in the epinephrine group, which achieved a mean DBP > 25 mm Hg during minutes 2–10 after resuscitation onset compared to the placebo group which had mean DBP < 25 mm Hg. However, we would have expected the animals resuscitated with epinephrine to achieve ROSC earlier as the mean DBP at 90 s was >25 mm Hg (Table 2). The most likely explanation is our definition of ROSC (HR > 100 bpm and MABP > 40 mm Hg). Animal studies of pediatric CA have found SBP > 90 mm Hg to be associated with increased survival with favorable neurologic outcome.46,47 We observed higher rates of ROSC in the epinephrine group, which had mean SBP > 90 mm Hg during minutes 2–10 after resuscitation onset, compared to the placebo group, which had mean SBP < 90 mm Hg. Higher DBP and SBP may not exclusively result from epinephrine administration but also from recovery of spontaneous circulation. We also analyzed hemodynamics during active resuscitation (minutes 2–3 after resuscitation onset) with exclusion of blood pressure values during the 30-s interval in which ROSC was achieved and forward, and we still found significantly increased DBP and SBP in animals resuscitated with epinephrine compared to placebo (Table 2).
We found no difference in brain MRS/MRI abnormality 6 h post ROSC based on Lac/NAA ratio, NAA/Cr ratio, DWI, or BOLD between animals resuscitated with epinephrine compared to placebo. The Lac/NAA ratio is known to increase during the secondary phase of energy failure between 6 and 24 h after cerebral hypoxia–ischemia.48 Thus, it is possible that we failed to capture the differences, i.e., both beneficial and adverse effects of epinephrine, due to the relatively short interval between CA and MRS. However, Zheng et al.49 showed that brain lactate levels peaked between 2 and 6 h following hypoxia–ischemia, and during our pilot studies, we performed both 6- and 12-h MRS/MRI examinations with no additional information gained. In addition, Tang et al.50 argue that pathophysiological changes after CA/hypoxia–ischemia, assessed by MRS/MRI, are more pronounced within the first 6 h after ROSC. Studies of adult CA have suggested that DWI-MRI can predict neurologic outcome as early as 3 h after ROSC.51,52 Our DWI results were in line with our MRS results that found no difference between the groups. This does not necessarily mean that a potential between-group difference would have settled at this timepoint. Accordingly, studies on long-term functional outcomes are needed. The majority of the animals had quite severe MRS/MRI findings; it is possible that we were unable to demonstrate any difference in MRS/MRI abnormality due to the severity of the insult. However, the model mimics a rare clinical condition where epinephrine is indicated with an a priori high risk of brain damage. A less severe insult would result in too many animals with spontaneous ROSC before drug administration.
We found no difference in the composite endpoint analysis of death or severe brain MRS/MRI abnormality. We decided not to use the clinical cut-off value of Lac/NAA > 0.39 to define severe brain MRS/MRI abnormality, as this value is extrapolated from human newborn studies and animal studies considerably different from our study with regards to timing of MRS; mean age = 5 days of life18 or even later,15 insult severity (not CA models), and MRS echo time (288 vs 135 ms).17,18,53 If our MRS results were equivalent to that of human infants, our findings would indicate severe brain damage with a high likelihood of adverse cognitive, motor, and language outcomes at 2 years of age.18 However, preclinical animal studies have shown that brain lactate measured by MRS decreases markedly while NAA increases between 6 and 72 h after a hypoxic–ischemic insult.49,50 The cut-off value to define severe brain damage should be higher when applied at 6 h compared to 72 h after the insult and beyond and when applied to CA models compared to HIE models. We therefore a priori chose to use a modified cut-off value of severe MRS/MRI abnormality.
This study has a number of limitations. (1) We used anesthetized and intubated animals. Anesthetics and drugs for pain relief are necessary for ethical reasons but may influence ROSC.54 However, all drugs were paused during CA and resuscitation. (2) Our study represents post-transitional neonatal hypoxic CA, and although newborn CA are most often hypoxic in origin, co-morbidities such as infection or hypovolemia exist.55,56 (3) The anatomy of the newborn pig is very similar to that of the newborn human. However, interspecies differences in vascular sensitivity to catecholamines likely exist, and caution must be exercised when translating to human neonates. (4) We delivered chest compressions asynchronous to ventilations, which is not in line with the current international neonatal resuscitation guidelines that recommends a synchronous technique. However, previous experimental animal models have shown that newborn piglets resuscitated with chest compressions performed asynchronous to ventilations have similar or improved ROSC and survival compared to piglets resuscitated with synchronized compressions and ventilations.57,58 (5) All animals received TH, which might postpone the processes involved in the brain injury beyond our observation period. However, no difference was found in a previous study of TH vs normothermia on brain injury by MRS/MRI at 6, 12, 24, and 72 h post ROSC.50 We initiated TH treatment right after achievement of ROSC, and target temperature was reached after 2 h (Fig. 1). According to current guidelines, TH should be commenced as early as possible within 6 h after birth.4 However, while TH in the clinical setting may be initiated later, we find the timing of 2 h realistic in many clinical settings.59,60,61 Regardless, interpretation of our results should consider that target temperature was reached at 2 h. (6) We used an MRS echo time of 135 ms for easy identification of lactate peaks due to peak inversion, and a small voxel to certify high spatial resolution. However, a small voxel decreases signal-to-noise ratio, which could challenge the detection of lactate peaks, as seen in WM. The early timing of MRS/MRI could affect lactate detection in WM. However, we previously measured Lac/NAA ratio in WM at 6, 12, and 24 h after hypoxia–ischemia and found a similar fraction of animals with absence of lactate in WM at 6 h compared to 12 and 24 h.21 A larger voxel and an echo time of 288 ms may help improve lactate detection in future studies. (7) The most important limitation to the neuroimaging is the few animals that survived to completion of the MRS/MRI examination. We were unable to include more animals in the study, as we had approval for only the prespecified number of animals from The Danish Animal Experiments Inspectorate based on the expected number of animals that would obtain ROSC.
Conclusion
We found that resuscitation with epinephrine compared to placebo improved the rate of ROSC after hypoxic CA in newborn piglets. We found no difference in time-to-ROSC. In animals resuscitated with epinephrine compared to placebo, 6-h survival was more than double. We found no difference in the composite endpoint of death or severe brain MRS/MRI abnormality. The remaining MRS/MRI results need confirmation in future studies as only six animals resuscitated with epinephrine and three animals resuscitated with placebo completed the MRS/MRI examination.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Perlman, J. M. et al. Part 7: Neonatal resuscitation 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations (reprint). Pediatrics 136, S120–S166 (2015).
Atkins, D. L. et al. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: Pediatric advanced life support. Pediatrics 117, e989-1004 (2006).
Wyckoff, M. H. et al. Part 13: Neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 132, S543–S560 (2015).
Kattwinkel, J. et al. Special Report - Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 126, e1400-13 (2010).
Madar, J. et al. European Resuscitation Council Guidelines 2021: newborn resuscitation and support of transition of infants at birth. Resuscitation 161, 291–326 (2021).
Schleien, C. L. et al. Effect of epinephrine on cerebral and myocardial perfusion in an infant animal preparation of cardiopulmonary resuscitation. Circulation 73, 809–817 (1986).
Mavroudis, C. D. et al. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit. Care 24, 1–13 (2020).
Kapadia, V. S. & Wyckoff, M. H. Epinephrine use during newborn resuscitation. Front. Pediatr. 5, 1–8 (2017).
Sun, S. et al. The effects of epinephrine on outcomes of normothermic and therapeutic hypothermic cardiopulmonary resuscitation. Crit. Care Med. 38, 2175–2180 (2010).
Ristagno, G. et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit. Care Med. 37, 1408–1415 (2009).
Perkins, G. D. et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N. Engl. J. Med. 379, 711–721 (2018).
Laptook, A. R. et al. Outcome of term infants using Apgar scores at 10 min following hypoxic-ischemic encephalopathy. Pediatrics 124, 1619–1626 (2009).
Harrington, D. J., Redman, C. W., Moulden, M. & Greenwood, C. E. The long-term outcome in surviving infants with Apgar zero at 10 min: a systematic review of the literature and hospital-based cohort. Am. J. Obstet. Gynecol. 196, 463.e1–463.e5 (2007).
Wyckoff, M. H. et al. Outcome of extremely low birth weight infants who received delivery room cardiopulmonary resuscitation. J. Pediatr. 160, 239.e2–244.e2 (2012).
Lally, P. J. et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol. 18, 35–45 (2019).
Thayyil, S. et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125, e382–95 (2010).
Alderliesten, T. et al. MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch. Dis. Child. Fetal Neonatal Ed. 102, F147–F152 (2017).
Mitra, S. et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch. Dis. Child. Fetal Neonatal Ed. 104, F424–F432 (2019).
Kilkenny, C., Browne, W., Cuthill, I. C., Emerson, M. & Altman, D. G. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579 (2010).
My Tech Tailor. Randomization tool: just flip a coin. (2010). https://justflipacoin.com.
Andelius, T. C. K. et al. No added neuroprotective effect of remote ischemic postconditioning and therapeutic hypothermia after mild hypoxia-ischemia in a piglet model. Front. Pediatr. 8, 1–10 (2020).
Fergusson, D., Aaron, S. D., Guyatt, G. & Hébert, P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. Br. Med. J. 325, 652–654 (2002).
Sobotka, K. S. et al. Effects of chest compressions on cardiovascular and cerebral hemodynamics in asphyxiated near-term lambs. Pediatr. Res. 78, 395–400 (2015).
Mendler, M. R. et al. Effect of different respiratory modes on return of spontaneous circulation in a newborn piglet model of hypoxic cardiac arrest. Neonatology 109, 22–30 (2016).
Solevåg, A. L., Dannevig, I., Nakstad, B. & Saugstad, O. D. Resuscitation of severely asphyctic newborn pigs with cardiac arrest by using 21% or 100% oxygen. Neonatology 98, 64–72 (2010).
Wagner, M. et al. Effects of epinephrine on hemodynamic changes during cardiopulmonary resuscitation in a neonatal piglet model. Pediatr. Res. 83, 897–903 (2018).
Linner, R., Werner, O., Perez-de-Sa, V. & Cunha-Goncalves, D. Early adrenaline administration does not improve circulatory recovery during resuscitation from severe asphyxia in newborn piglets. Resuscitation 83, 1298–1303 (2012).
McNamara, P. J., Engelberts, D., Finelli, M., Adeli, K. & Kavanagh, B. P. Vasopressin improves survival compared with epinephrine in a neonatal piglet model of asphyxial cardiac arrest. Pediatr. Res. 75, 738–748 (2014).
Andersen, L. W. et al. Time to epinephrine and survival after pediatric in-hospital cardiac arrest. JAMA 314, 802–810 (2015).
Matsuyama, T. et al. Pre-hospital administration of epinephrine in pediatric patients with out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 75, 194–204 (2020).
American Heart Association & American Association of Pediatrics. Textbook of Neonatal Resuscitation 7th edn (2016).
Sankaran, D. et al. Effect of a larger flush volume on bioavailability and efficacy of umbilical venous epinephrine during neonatal resuscitation in ovine asphyxial arrest. Children 8, 464 (2021).
Pasquin, M. P. et al. Comparison of different compression to ventilation ratios (2: 1, 3: 1, and 4: 1) during cardiopulmonary resuscitation in a porcine model of neonatal asphyxia. Neonatology 114, 37–45 (2018).
Schmölzer, G. M. et al. Cardiopulmonary resuscitation with chest compressions during sustained inflations: a new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation 128, 2495–2503 (2013).
Kleinman, M. E., Oh, W. & Stonestreet, B. S. Comparison of intravenous and endotracheal epinephrine during cardiopulmonary resuscitation in newborn piglets. Crit. Care Med. 27, 2748–2754 (1999).
Schleien, C. L., Koehler, R. C., Shaffner, D. H., Eberle, B. & Traystman, R. J. Blood-brain barrier disruption after cardiopulmonary resuscitation in immature swine. Stroke 22, 477–483 (1991).
Hassan, M. A. et al. Reliability of pulse oximetry during progressive hypoxia, cardiopulmonary resuscitation, and recovery in a piglet model of neonatal hypoxic cardiac arrest. Neonatology 112, 40–46 (2017).
Goto, Y., Funada, A. & Goto, Y. Subsequent shockable rhythm during out-of-hospital cardiac arrest in children with initial non-shockable rhythms: a nationwide population-based observational study. J. Am. Heart Assoc. 5, 1–11 (2016).
Lelovas, P. P., Kostomitsopoulos, N. G. & Xanthos, T. T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 53, 432–438 (2014).
Kern, K. B., Ewy, G. A., Voorhees, W. D., Babbs, C. F. & Tacker, W. A. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation 16, 241–250 (1988).
Sanders, A. B., Ewy, G. A. & Taft, T. V. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit. Care Med. 12, 871–873 (1984).
Paradis, N. A. et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 263, 1106–1113 (1990).
Niemann, J. T., Criley, J. M., Rosborough, J. P., Niskanen, R. A. & Alferness, C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann. Emerg. Med. 14, 521–528 (1985).
O’Brien, C. E. et al. Association of diastolic blood pressure with survival during paediatric cardiopulmonary resuscitation. Resuscitation 143, 50–56 (2019).
Berg, R. A. et al. Association between diastolic blood pressure during pediatric in-hospital cardiopulmonary resuscitation and survival. Circulation 137, 1784–1795 (2018).
Lautz, A. J. et al. Hemodynamic-directed cardiopulmonary resuscitation improves neurologic outcomes and mitochondrial function in the heart and brain. Crit. Care Med. 47, e241–e249 (2019).
Senthil, K. et al. Haemodynamic-directed cardiopulmonary resuscitation promotes mitochondrial fusion and preservation of mitochondrial mass after successful resuscitation in a pediatric porcine model. Resuscitation 6, 100124 (2021).
Hassell, K. J., Ezzati, M., Alonso-Alconada, D., Hausenloy, D. J. & Robertson, N. J. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch. Dis. Child. Fetal Neonatal Ed. 100, F541–F551 (2015).
Zheng, Y. & Wang, X. M. Expression changes in lactate and glucose metabolism and associated transporters in basal ganglia following hypoxic-ischemic reperfusion injury in piglets. Am. J. Neuroradiol. 39, 569–576 (2018).
Tang, Z.-R. et al. Effects of hypothermia on brain injury assessed by magnetic resonance imaging after cardiopulmonary resuscitation in a porcine model of cardiac arrest. Am. J. Emerg. Med. 31, 86–93 (2013).
Jeon, C. H. et al. Comparison of brain computed tomography and diffusion-weighted magnetic resonance imaging to predict early neurologic outcome before target temperature management comatose cardiac arrest survivors. Resuscitation 118, 21–26 (2017).
Park, J. S. et al. Efficacy of diffusion-weighted magnetic resonance imaging performed before therapeutic hypothermia in predicting clinical outcome in comatose cardiopulmonary arrest survivors. Resuscitation 88, 132–137 (2015).
Pang, R. et al. Proton magnetic resonance spectroscopy lactate/n-acetylaspartate within 48 h predicts cell death following varied neuroprotective interventions in a piglet model of hypoxia-ischemia with and without inflammation-sensitization. Front. Neurol. 11, 883 (2020).
Kurita, T., Morita, K., Kazama, T. & Sato, S. Comparison of isoflurane and propofol-fentanyl anaesthesia in a swine model of asphyxia. Br. J. Anaesth. 91, 871–877 (2003).
Aziz, K., Chadwick, M., Baker, M. & Andrews, W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation 79, 444–452 (2008).
Randis, T. M. et al. Incidence of early-onset sepsis in infants born to women with clinical chorioamnionitis. J. Perinat. Med. 46, 926–933 (2018).
Schmölzer, G. M. et al. 3:1 compression to ventilation ratio versus continuous chest compression with asynchronous ventilation in a porcine model of neonatal resuscitation. Resuscitation 85, 270–275 (2014).
Aggelina, A. et al. Continuous chest compressions with asynchronous ventilation improve survival in a neonatal swine model of asphyxial cardiac arrest. Am. J. Emerg. Med. 48, 60–66 (2021).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Azzopardi, D. et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 15, 145–153 (2016).
Reaney, L., Livingstone, V., Bogue, C., Dempsey, E. M. & Filan, P. M. Timing of therapeutic hypothermia for inborn and outborn infants with neonatal encephalopathy. Ir. Med. J. 109, 369 (2016).
Acknowledgements
We wish to thank the staff at the animal research facilities at Institute of Clinical Medicine, Aarhus University.
Funding
The study was supported by the Elsass Foundation (Grant nr: 17-3-1448) and Aarhus University.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements. Each author completed the following criteria: (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by The Danish Animal Experiments Inspectorate, Directive 2010/63/EU (license no: 2018-15-0201-01555). Experiments were performed in accordance with national guidelines on animal welfare. Reporting complies with the ARRIVE 2.0 guidelines. This preclinical animal study did not include patients or require patient consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Andersen, H.B., Andersen, M., Andelius, T.C.K. et al. Epinephrine vs placebo in neonatal resuscitation: ROSC and brain MRS/MRI in term piglets. Pediatr Res 93, 511–519 (2023). https://doi.org/10.1038/s41390-022-02126-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02126-4
This article is cited by
-
Is epinephrine effective during neonatal resuscitation?
Pediatric Research (2023)