Abstract
Background
Neuroprognostication in neonates with neonatal encephalopathy (NE) may be enhanced by early serial measurement of a panel of four brain-specific biomarkers.
Methods
To evaluate serum biomarkers, 40 NE samples and 37 healthy neonates from a biorepository were analyzed. Blood samples were collected at 0–6, 12, 24, 48, and 96 h of life. MRI provided a short-term measure of injury. Long-term outcomes included death or a Bayley III score at 17–24 months of age.
Results
Glial fibrillary acidic protein (GFAP), ubiquitin c-terminal hydrolase-L1 (UCH-L1), and Tau peaked at 0–6 h of life, while neurofilament light chain (NFL) peaked at 96 h of life. These four marker concentrations at 96 h of life differentiated moderate/severe from none/mild brain injury by MRI, while GFAP and Tau showed early discrimination. For long-term outcomes, GFAP, NFL, Tau, and UCH-L1 could differentiate a poor outcome vs good outcome as early as 0–6 h of life, depending on the Bayley domain, and a combination of the four markers enhanced the sensitivity and specificity. Machine learning trajectory analyses identified upward trajectory patients with a high concordance to poor outcomes.
Conclusion
GFAP, NFL, Tau, and UCH-L1 may be of neuroprognostic significance after NE.
Impact
-
Serial measurements of GFAP, NFL, Tau, and UCH-L1 show promise in aiding the bedside clinician in making treatment decisions in neonatal encephalopathy.
-
The panel of four neuroproteins increased the ability to predict neurodevelopmental outcomes.
-
The study utilized a trajectory analysis that enabled predictive modeling.
-
A panel approach provides the bedside clinician with objective data to individualize care.
-
This study provides the foundation to develop a point of care device in the future.
Similar content being viewed by others
Introduction
Neonatal encephalopathy (NE) is a significant cause of morbidity and mortality in neonates. The incidence of NE ranges from 1 to 8 per 1000 live births in developed countries to as high as 26 per 1000 live births in developing countries.1 Therapeutic hypothermia improves the neurodevelopmental outcome in infants with moderate NE due to hypoxia-ischemia.2,3,4,5
Current monitoring and evaluation of NE, outcome prediction, and efficacy of hypothermia treatment rely on a combination of the bedside neurological exam, cranial ultrasound, brain magnetic resonance imaging (MRI), and electroencephalography (EEG).6,7,8,9,10 However, the clinical monitoring and evaluations currently used do not adequately identify short- or long-term outcomes during the initial 48–72 h of hypothermia therapy.11,12 Therefore, the development of a simple, inexpensive, non-invasive, rapid biomarker test is essential to identify candidates for therapeutic hypothermia and to assess outcomes.
Serum biomarkers offer the potential to identify injury and response to therapy during the 72 h of hypothermia. Our group has investigated ubiquitin c-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP), and these biomarkers demonstrate great promise.13,14 UCH-L1 is abundant in neurons and plays a critical role in cellular protein degradation during normal and pathological conditions.15 UCH-L1 is upregulated following hypoxia-ischemia by hypoxia-inducible factor 1α and 2α.16 GFAP is a type III intermediate filament that forms part of the cytoskeleton of mature astrocytes and other glial cells but is not found outside the CNS.17 CNS injury produces gliosis that results in an up-regulation of GFAP expression. Thus, GFAP is a good biomarker candidate for brain injury screening. Other potential biomarker proteins for neonates undergoing hypothermia for NE include neurofilament light chain (NFL) and Tau. NFL is a CNS-enriched neurofilament protein with a weight of 61.5 kDa protein and is predominantly expressed in myelinated subcortical axons.18 Tau, a microtubule-binding protein, is found mainly in the cytoplasm.19 In neurons, Tau is found mainly in the axon, although localization in the somatodendritic compartment, including the dendritic spines, has also been described.19
An approach utilizing a panel of serum protein biomarkers may provide the bedside clinician with more useful information than observing a single biomarker. During initial assessment and treatment, a panel may enhance the ability of the clinician to identify the timing of hypoxic-ischemic injury and the patient’s location on the pathophysiologic cascade. The panel may also provide the clinician with information about the response to therapy, identify regions or patterns of brain injury, and predict short-term injury on MRI and long-term neurodevelopmental outcomes. For these potential clinical reasons and based on recent studies in adults utilizing this biomarker panel for detection of traumatic brain injury, our group chose to study a panel of four biomarkers,20 UCH-L1, GFAP, NFL, and Tau, in infants with NE undergoing hypothermia.
We hypothesized that elevated concentrations of the biomarkers in the serum would correspond to the severity of brain injury detected by MRI and neurodevelopmental impairments at 17–24 months. In addition, using a novel approach, we combined all four biomarkers to examine whether the combination or the temporal profile/trajectory of the combination improved the ability to predict MRI injury severity and neurologic outcomes.
Methods
Patient populations
NE subjects
The University of Florida Institutional Review Board approved the study. All parents of neonates eligible for therapeutic hypothermia were consented within 72 h of birth for entry into the Florida Neonatal Neurologic Network registry and biorepository as previously reported.21,22 Data were extracted from the electronic medical records and were recorded in the REDCap electronic data capture tools.23 For this study, a retrospective analysis of neuroproteins from select specimens in the data registry and biorepository was conducted. From each patient in the NE group, a maximum of five samples were collected from the time of birth as follows: between 0 and 6 h (time 1), 12 h (time 2), 24 h (time 3), 48 h (time 4), and 96 h (time 5). These timepoints coincided with the time before hypothermia or at the initiation of hypothermia, during hypothermia, and after the hypothermic treatment.
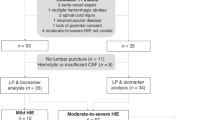
Specimens were selected by NE outcome defined by MRI results. Twenty specimens from subjects with NE with no or mild brain injury were compared to 20 specimens from subjects with NE with moderate or severe brain injury. Of the 40 total subjects with NE, 24 had outcome data available in the form of either Bayley testing at 17–24 months of age (20 subjects) or were deceased (4 subjects). All subsequently deceased subjects received redirection of care on days 5, 6, 20, and 4 months after birth.
Control subjects
Thirty-seven healthy neonates were evaluated to ensure they met eligibility criteria for controls (Apgar scores of 8 or higher at 1 and 5 min of life, no evidence of encephalopathy, and admission to the newborn nursery). At the time of birth, one blood sample was collected from each patient’s umbilical cord. Control specimens were collected during the same time period as the neonates with NE. These control specimens were obtained for analysis from the biorepository.21,22
Blood processing
Serum samples (1 ml) were collected using a 3.5 ml serum separator tube (BD Vacutainer® SST Plus Blood Collection Tube, Franklin Lakes, NJ) and processed per the laboratory protocol previously reported.14
Biomarker analysis—enzyme-linked immunosorbent assay
Investigators blinded to the clinical data used a SIOMA Neuro 4 Plex kit in an SR-X immunoassay analyzer (Quanterix Corp, Boston, MA) to measure the concentrations of GFAP, UCH-L1, NFL, and Tau.
MRI scoring
MRI was performed at either 4–5 days of age (n = 33) following rewarming or 7–12 days of age (n = 6) if the neonates were not stable enough for transport to the MRI machine at 4–5 days of age. One MRI was done at 50 days of life for a patient that was unstable due to comorbidities. Neonates were imaged on a 3T scanner (Siemens, Malvern, PA). The analysis focused on the T1-weighted, T2-weighted, and diffusion-weighted imaging abnormalities. Two blinded subspecialty board-certified neuroradiologists with over 10 years of experience interpreted all of the MRI images using the Barkovich scoring system.24 Individual brain regions scored included the basal ganglia (0–4), the watershed cortex/white matter (0–5), and combined basal ganglia/watershed (0–4). Infants with scores of 0–2 in any region were categorized as no/mild injury, and infants with scores equal to or greater than 3 in any region were categorized as moderate/severe injury.
Outcome assessment (Bayley III Testing at 17–24 months of age)
All patients with NE are followed in our Early Developmental Assessment clinic and receive a Bayley III exam as part of their routine clinical care. We analyzed 20 subjects ranging in age from 17 to 24 months (mean 20 months) who had available biomarker data and Bayley III exams. The analysis also included four subjects who were deceased. These deceased subjects were included in the poor outcome categories for the neurodevelopmental outcome. Therefore, 24 of the 40 original cohort subjects had long-term outcome data defined as a Bayley score or death. Individual developmental domains on the Bayley III, including motor, cognitive, and language, were analyzed. The analysis was performed using normal-theory simple linear regressions (linear models) to relate the biomarker concentrations and the Bayley domain scores. Logistic regressions with one covariate (generalized linear models) were used to relate the binary responses to the biomarkers. A good outcome was identified when a Bayley domain score was 85 or greater, and a poor outcome was identified when a Bayley score was less than 85 or death occurred. To determine the ability of these biomarkers to predict the good versus poor developmental outcomes at 17–24 months of age, prognostic models for developmental outcomes were derived for all biomarkers (Supplementary Table 2).
Data management and statistical analysis
One-way analysis of variance was used to assess whether the mean concentrations differed among the groups for each of the timepoints.
To attain normal distribution, all serum biomarker concentrations were logarithmically transformed. Mann–Whitney U and Kruskal–Wallis tests were conducted to assess the difference between groups. For categorical variable comparisons, χ2 with Fisher’s test was used. To evaluate whether biomarker levels can distinguish between good and poor outcome, we conducted the receiver operating characteristic (ROC) and area under the curve (AUC) analyses. Two-tailed tests were used to determine significance at the 5% level, and the analysis was performed in SPSS version 21.0.
Unlike traditional latent cure analysis and hierarchical modeling, the estimated covariates in trajectory analysis included both trajectory shape and groups of individuals with similar phenomena over time.25 SAS (v 9.4, Cary, NC) and RStudio 1.0 statistical software were used for trajectory analyses. We used unconditional LCMM to model the four biomarkers’ combined trajectories over time. Under each trajectory class, patients were classified into distinct latent trajectory groups. The only variables used to infer trajectory groups were subject, combined biomarker levels (ratios to the 0–6 h data point), and time. Akaike information criterion (AIC) and Bayesian information criteria (BIC) were used to evaluate the goodness of model fit. Considering the small sample size, we compared the two-class model versus the three-class model, and the two-class model reached the inflection point of the AIC and BIC. ROC curves for the individual biomarkers as well as for a combination of the four markers over time in patients with varying MRI injury are found in Supplementary Table 1.
The above analysis was repeated in a cohort of neonates with sentinel events.
Results
Patient demographics and study profile
The study population consisted of primarily males (65%) with a mean gestational age of 38.3 weeks (SD ±1.9) and a mean birth weight of 3340 g (SD ±783). Patient characteristics at enrollment were analyzed by no/mild and moderate/severe brain injury on MRI. All characteristics were similar between the groups except for the Apgar scores at 5 min of life and the Sarnat scores (P < 0.05). The Apgar scores were lower in the moderate/severe brain injury on MRI group, and more infants in the moderate/severe brain injury on MRI group had a stage III initial Sarnat exam. The time to initiate hypothermia was not different between the no/mild brain injury group (3.187 ± 1.67 h after birth) and the moderate/severe brain injury group (2.6 ± 1.52 h after birth). Additional characteristics and details are shown in Table 1.
Neuroprotein biomarker time profile in infants with NE compared to controls
Log-scale median and interquartile range serum concentrations of GFAP, NFL, UCH-L1, and Tau over time are in Fig. 1. Serum concentrations of GFAP and NFL increased in infants with NE undergoing hypothermia compared to controls at all timepoints (P < 0.05). GFAP peaked at 0–6 h and NFL peaked at 96 h. UCH-L1 (P < 0.05) and Tau (P < 0.01) serum concentrations in patients with NE undergoing hypothermia increased only at 0–6 h compared to control samples.
Biomarker concentrations and correlation with MRI injury
The injury severity score of the basal ganglia (A), watershed (B), and basal ganglia/watershed (C) were recorded (Fig. 2). Compared to the control serum concentrations of GFAP, serum concentrations in NE infants undergoing hypothermia increased within 6 h after birth regardless of the MRI injury group in all three brain regions. The GFAP serum concentration increased at 48 and 96 h of age in neonates with moderate/severe brain injury on MRI compared to neonates with no/mild injury in all three brain regions scored (P < 0.05). The UCH-L1 serum concentrations increased in neonates with moderate/severe brain injury compared to no/mild injury at 96 h of life in all three brain regions (P < 0.05). The NFL concentrations increased at 96 h in neonates with moderate/severe injury compared to no/mild injury in the basal ganglia and watershed brain regions (P < 0.05). Tau increased in the moderate/severe group at 48 and 96 h in all three brain regions (P < 0.05). In addition, Tau increased in the moderate/severe group compared to the no/mild group at 12 h of age in the watershed brain region (Fig. 2). GFAP, UCH-L1, NFL, and Tau concentrations at all timepoints showed varying degrees of discrimination (all AUC > 0.5). The combination of the four markers increased the AUC (blue) compared to the individual AUC and reached a statistically significant difference after 6 h of life.
a Top panel: MRI injury score for basal ganglia. b Mid panel: MRI injury score for watershed region. c Bottom panel: MRI injury score for basal ganglia/watershed regions. The scores of lesions at basal ganglia, watershed, thalamus, and cortex were calculated. *P < 0.05, **P < 0.01, the comparison was between the neonates without injury or mild injury (MRI score = 0–2) and those with moderate and severe injury (MRI score = 3–5) (n = 40).
Biomarker concentrations and correlation with neurologic outcomes
A representative ROC for hour 12 is depicted in Fig. 3. Serum GFAP, NFL, Tau, and UCH-L1 levels showed varying degrees of discrimination with an AUC value of 0.772, 0.574, 0.753, and 0.784, respectively, in cognitive function, 0.741, 0.635, 0.759, and 0.806 in language function, 0.53, 0.524, 0.542, and 0.613 in motor function between patients with good versus poor outcomes. The combination of the four biomarkers increased the prognostic ability with an AUC 0.883 in cognitive function, AUC 0.841 in language function, and AUC 0.75 in motor function. In Supplementary Fig. 1, log-scale biomarker concentrations were plotted between the good and poor outcomes for each Bayley domain. The GFAP concentrations were elevated at the sample collection times of 12, 24, 48, and 96 h in patients with poor cognitive outcomes compared to patients with good outcomes at 17–24 months (P < 0.05). UCH-L1 and Tau concentrations increased in patients with poor cognitive outcomes compared to patients with good outcomes at the 24-, 48-, and 96-h sampling timepoints (P < 0.05), while NFL increased in the poor outcome group at 48 and 96 h (P < 0.05). Increased serum concentrations of UCH-L1 at 0–6, 24 and 96 h (P < 0.05), NFL at 48 and 96 h (P < 0.05), and Tau at 24, 48 and 96 h (P < 0.05) were associated with poor outcomes in the language domain. Poor outcomes in the motor domain were associated with increased concentrations of UCH-L1 at 48 h (P < 0.05).
Composite biomarker trajectory analysis of neurologic outcomes
Four-composite biomarker scores were derived using relative biomarker concentration changes from baseline (0–6 h) at each sample collection timepoint (expressed as a ratio). Figure 4a shows a four-marker standardized composite score (GFAP, NFL, UCH-L1, Tau; mean + SEM) over time for class. Patients with good outcomes showed slightly increased composite scores while the four-marker composite score for patients with poor outcomes rose sharply from 24 h of life. After best trajectory mode fit comparison, low trajectory (class 1, blue) and high trajectory (class 2, red) were identified (Fig. 4b). Based on the four-marker score, 25% of the patients were classified into the high-trajectory group, and 75% were classified into the low-trajectory group. Figure 4c showed complete concordance of group membership in independently predicting patient outcome. Follow-up at 17–24 months showed that all patients in the high-trajectory group (class 2) were classified as the poor neurological outcome group while 90% of the low-trajectory patients (class 1) were classified to the good neurological outcome group. The odds ratios for poor cognitive, language, and motor outcomes in the composite high-trajectory group (class 2) were 2.57 (95% CI: 0.914–7.23; z statistics 1.79, significance level at P = 0.0435), 2.12 (95% CI: 0.776–5.81; z statistics 1.47, significance level at P = 0.14), and 0.92 (95% CI: 0.353–2.413; z statistics 1.632, significance level at P = 0.87), respectively. The respective biomarker concentrations in each trajectory class over time are shown in Supplementary Fig. 2. A significant difference in biomarker concentrations is shown from 24 h for GFP, NFL, UCH-L1, Tau, and the four-marker composite scores.
Group trajectory is indicated by the solid line; 95% confidence intervals are indicated by dotted lines. Four markers 0h-standardized score based latent trajectories. Temporal biomarker levels are standardized with levels at 0 h as 100. a Two-group biomarker trajectory profiles using concentration change from baseline (0 h). The y-axis represents the natural log back-transformed concentration ratio change from baseline. b All patients in the high-trajectory group (class 2) belong to the poor cognitive function group, while 90% of the low-trajectory patients (class 1) belong to the good cognitive function group. 85% of patients in the low-trajectory group have good outcomes in language function. c Logistic regression modeling predicting NE outcomes using biomarker trajectory groups. Odds ratios are expressed using group 1 as the reference group.
Biomarker concentrations in neonates with sentinel events
A subgroup analysis of biomarker concentrations from neonates with sentinel events (n = 14) was performed and compared to concentrations in neonates without sentinel events (n = 26) (Fig. 5). NFL serum concentrations at 96 h of age increased in neonates with sentinel events compared to those without (P < 0.05). UCH-L1 serum concentrations at 96 h of age increased in neonates with a sentinel event compared to neonates without a sentinel event (P < 0.05). Next, neonates with sentinel events were divided into two groups to identify any differences between the serum concentrations of each group and the MRI injury severity (no/mild and moderate/severe) (Fig. 6). Also, neonates with non-sentinel events were divided using the same scheme with MRI injury severity (no/mild and moderate/severe).
Neuroprotein biomarker concentrations in neonates with a sentinel event and moderate/severe injury to the basal ganglia (a), the watershed (b), the basal ganglia and watershed (c). MRI injury scores for neonates with no/mild injury (MRI score = 0–2) differed from neonates with moderate/severe injury (MRI score = 3–5). *P < 0.05, **P < 0.01.
With respect to the MRIs, neonates with sentinel events and moderate/severe injury to the basal ganglia (n = 7) had increased serum concentrations of GFAP at 48 h, Tau at 48 and 96 h, and UCH-L1 at 48 and 96 h compared to neonates with sentinel events and no/mild injury (n = 7, P < 0.05). Neonates with moderate/severe injury (n = 8) to the watershed regions had increased serum concentrations of GFAP at 12, 24, and 48 h, Tau at 48 and 96 h and UCH-L1 at 48 h after birth compared to neonates with sentinel events and no/mild injury (n = 6, P < 0.05). Finally, serum concentrations of NFL, Tau, and UCH-L1 increased in neonates with moderate/severe injury (n = 7) to the basal ganglia/watershed brain regions compared to neonates with sentinel events and no/mild injury (n = 7, P < 0.05). Neonates without sentinel events and no/mild injury were similar to neonates without sentinel events that had moderate/severe injury (n = 26).
Discussion
In this study, four serum biomarkers were examined. This study is novel in that it examined both a short-term marker of brain injury, MRI, and a long-term marker, neurodevelopmental outcomes. The panel of four biomarkers, when the results were combined, increased the AUC in predicting long-term neurodevelopmental outcomes at 17–24 months of age. To the best of our knowledge, using a panel of biomarkers combined to improve the ability to predict long-term neurologic outcomes is a unique approach. Trajectory analysis has been used in pediatric biomarker studies, but to the best of our knowledge, this is the first time that this method has been applied to neonates and yielded novel observations.25
UCH-L1 has been studied in NE. A recent study by Massaro et al. demonstrated an association between UCH-L1 concentrations at less than 24 h of age and the severity of brain injury visible on MRI.26 The same study did not show an association between UCH-L1 concentrations and neurodevelopmental outcomes at 12 months of age.26 A potential explanation for the lack of correlation with developmental outcomes at 12 months of age may be due to the wider variability in sample collection time, ranging from 0–24 h. As our current data and previous publication have demonstrated, UCH-L1 serum concentrations peak at 0–6 h and undergo a rapid decrease between 12 and 24 h of age, and the wide variation in the timing of the sample collection by Massaro et al. may have diluted the effect on predicting long-term outcomes.13 Similar to the Massaro study, Chalak et al. did not find an association with neurodevelopmental outcomes when UCH-L1 was measured at 6–24 h of hypothermia.27 Our group has previously studied UCH-L1 in neonates with NE undergoing hypothermia and demonstrated that serum concentrations at 0–6 and 12 h demonstrated promise in differentiating a good outcome from a poor outcome utilizing the same definitions for good versus poor outcomes.13 Our current and previous studies suggest that UCH-L1 serum concentrations before 48 h of life are associated with MRI injury and long-term neurologic outcomes.
Increased GFAP serum concentrations sampled at 12, 48, and 96 h of life were associated with a poor outcome in the cognitive domain. GFAP has also been widely studied in NE.27,28,29,30 Ennen et al. found that the serum concentrations of GFAP in 23 neonates undergoing hypothermia were increased compared to control patients at 0–6, 24, and 72 h of age and 1 day post cooling.28 The study also compared the concentrations of serum GFAP with normal and abnormal MRI. Increased serum concentrations at 24 and 48 h and post-cooling days 1, 2, 3, and 4 were associated with an abnormal MRI. Massaro et al. examined the GFAP serum concentrations in 20 neonates with NE undergoing hypothermia at 0, 12, 24, and 72 h of cooling and compared the results to brain injury as measured by MRI.29 Increased serum concentrations of GFAP at 24 and 72 h of cooling were associated with death or severe MRI injury.29 Chalak et al. demonstrated that increased GFAP concentrations at 6–24 h of cooling in neonates with NE were associated with abnormal neurologic outcomes at 15–18 months of age.27 Our results and those from the literature suggest that GFAP has the potential to be a useful clinical biomarker in NE with the predictive value occurring 24 h after birth and beyond.
Plasma NFL concentrations measured in neonates undergoing therapeutic hypothermia are associated with unfavorable outcomes as measured by MRI and increase over time.31 Our study, which used serum, was similar in finding that NFL rises over time and a later timepoint, 96 h of age, corresponded to MRI injury. Due to the lack of data on NFL, the neuroprotein should be explored further in a larger study.
Tau has previously been associated with developmental outcomes at 1 year of age when measured at or before 24 h of life in neonates undergoing hypothermia,26 and serum concentrations correspond to the clinical severity of infants with NE.32 Our results demonstrated that increased serum concentrations of Tau measured at 24, 48, and 96 h of age were associated with poor neurodevelopmental outcomes in the domains of language and cognition.
By combining a panel of biomarkers, the serum biomarker concentrations after birth, the short-term outcome of injury on MRI, and the long-term neurodevelopmental outcome at 17–24 months of age may have improved predictive value. With respect to the short-term outcomes of MRI injury, a combination of all four biomarkers increased the AUC at 12 and 48 h in predicting injury to the basal ganglia, the AUC at 48 h in predicting injury to the watershed region, and the AUC at 12 and 48 h in predicting injury to the thalamus/basal ganglia/watershed regions. The association with long-term neurodevelopmental outcomes was also improved by combining all four biomarkers. An increased AUC at 12, 24, 48, and 96 h of age was associated with the cognitive domain, an increased AUC at 0–6, 12, 24, 48, and 96 h was associated with the language domain, and an increased AUC at 24 and 48 h of age was associated with the motor domain. Many of the above timepoints associated with outcomes are during the first 24 h of age when clinical decision making is occurring with respect to neurointensive care interventions. These early timepoints may enable the clinician to gauge the degree of injury while the later timepoints may allow the bedside team to determine the effect of the clinical intervention in preventing late biomarker peaks. We envision an online tool in which the results from the biomarkers, the neurologic exam, and the aEEG or EEG background are entered into a calculator, which will give the clinician a predictive percentage of the patient developing a poor long-term neurodevelopmental outcome. This predictive percentage would provide the clinician with objective information to assist with the decision to offer the family redirection of care. The calculator would be dynamic so values could be entered at each sampling timepoint.
Despite major advances in biomarker research in NE prognosis estimation, several challenges still exist in effectively modeling biomarker-outcome relationships. Traditionally, biomarker data collected over time are summarized into single-point estimates. Then, these point estimates are used to examine the relationship between biomarker levels and the outcome of interest. However, changes over time may reveal short-term injury and long-term neurodevelopmental outcomes. Due to the problems associated with the traditional single-point biomarker analysis, we have chosen in this aim to examine the temporal/trajectory profile for the biomarkers. Trajectory analysis is a specialized application of finite mixture modeling that determines trends in longitudinally collected data by identifying trajectory groups on a likelihood basis and does not rely solely on mean averages or peak concentrations of biomarkers.25 Essentially, this technique allows testing of temporal patterns of biomarkers over several days to identify distinctive trajectories.25
A major weakness with our design was the utilization of a convenience sample. We randomly selected neonates with known MRI findings in a retrospective fashion from our biorepository to make the groups equal in number with respect to the number of neonates with no/mild injury and moderate/severe injury. Another weakness with neonatal biomarker studies such as our own is the lack of studying neonates with only sentinel events. Sentinel events are known events that are associated with infants with NE and include placental abruptions, uterine ruptures, and shoulder dystocia and occur in 15–29% of cases of NE.33,34 Sentinel events allow for the exact timing of the HI insult. This exact timing is important because biomarker concentrations change over time, and, unlike adults with traumatic brain injury (TBI) or stroke, the exact timing of injury in NE may be unknown. This is a major confounding variable that has not been accounted for in the NE biomarker literature. This confounder may have diluted the peaks of the biomarkers by including neonates who may have been beyond 6 h into the injury cascade at the time of the first serum sample. We attempted to account for this confounding variable by performing a subgroup analysis of all neonates with sentinel events; however, the sample size was small. Another weakness is the lack of follow-up on all of the neonates in the study. Neurodevelopmental follow-up data at 17–24 months of age was available for only 55% of the neonates enrolled in the study who survived to 17–24 months of age. Finally, subjective reading was used for analyzing the MRI results, and the timing of obtaining the MRIs was not the same in all neonates. Ideally, objective readings with volumetric measurement of the injury should be used to correlate injury with the serum concentrations of biomarkers, and the study should be performed on the same day after birth in all neonates to reduce confounding variables.
Conclusion
Our data demonstrate four biomarkers that are associated with short-term MRI injury and long-term neurodevelopmental impairments at 17–24 months of age. Future investigations should include multiple study sites to increase subject numbers, earlier timepoints, and prospective patient populations with known sentinel events to understand the biomarker changes over time. Understanding the temporal relationship of biomarker concentrations to neonatal neurologic injury is critically important because adult trials of traumatic brain injury are working on similar bedside devices to obtain near real-time results of UCH-L1 and GFAP. This advancement in adult medicine offers the potential of using these biomarkers at the bedside of neonates with NE undergoing hypothermia.
References
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Wyatt, J. S. et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 119, 912–921 (2007).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med 361, 1349–1358 (2009).
Tagin, M. A., Woolcott, C. G., Vincer, M. J., Whyte, R. K. & Stinson, D. A. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 166, 558–566 (2012).
Garfinkle, J. et al. Cooling in the real world: therapeutic hypothermia in hypoxic-ischemic encephalopathy. Eur. J. Paediatr. Neurol. 17, 492–497 (2013).
Leijser, L. M. et al. Prediction of short-term neurological outcome in full-term neonates with hypoxic-ischaemic encephalopathy based on combined use of electroencephalogram and neuro-imaging. Neuropediatrics 38, 219–227 (2007).
Jouvet, P. et al. Reproducibility and accuracy of mr imaging of the brain after severe birth asphyxia. AJNR Am. J. Neuroradiol. 20, 1343–1348 (1999).
Boichot, C. et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology 239, 839–848 (2006).
van Laerhoven, H., de Haan, T. R., Offringa, M., Post, B. & van der Lee, J. H. Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics 131, 88–98 (2012).
Bonifacio, S. L., Glass, H. C., Peloquin, S. & Ferriero, D. M. A new neurological focus in neonatal intensive care. Nat. Rev. Neurol. 7, 485–494 (2011).
Gunn, A. J. et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J. Pediatr. 152, 55–58.e51 (2008) .
Thoresen, M., Hellstrom-Westas, L., Liu, X. & de Vries, L. S. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 126, e131–e139 (2010).
Douglas-Escobar, M. V. et al. Uch-L1 and Gfap serum levels in neonates with hypoxic-ischemic encephalopathy: a single center pilot study. Front Neurol. 5, 273 (2014).
Douglas-Escobar, M. et al. A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatr. Res. 68, 531–536 (2010).
Day, I. N. & Thompson, R. J. Uchl1 (Pgp 9.5): neuronal biomarker and ubiquitin system protein. Prog. Neurobiol. 90, 327–362 (2009).
Hongwei, W., Wang, Y., Wang, W., Li, W. & Feng, X. Hif1α and Hif2α mediated Uchl1 upregulation in hypoxia-induced neuronal injury following neuronal hypoxic ischemic encephalopathy. Int J. Clin. Exp. Pathol. 9, 2677–2685 (2016).
Eng, L. F. Glial fibrillary acidic protein (Gfap): the major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 8, 203–214 (1985).
Shahim, P., Zetterberg, H., Tegner, Y. & Blennow, K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 88, 1788–1794 (2017).
Avila, J. Our working point of view of Tau protein. J. Alzheimers Dis. 62, 1277–1285 (2018).
Czeiter, E. et al. Blood biomarkers on admission in acute traumatic brain injury: relations to severity, CT findings and care path in the center-TBI study. EBioMedicine 56, 102785 (2020).
Chang, L. L. et al. Enteral feeding as an adjunct to hypothermia in neonates with hypoxic-ischemic encephalopathy. Neonatology 113, 347–352 (2018).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med 353, 1574–1584 (2005).
Harris, P. A. et al. Research Electronic Data Capture (Redcap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Barkovich, A. J. et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am. J. Neuroradiol. 19, 143–149 (1998).
Berger, R. P., Bazaco, M. C., Wagner, A. K., Kochanek, P. M. & Fabio, A. Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev. Neurosci. 32, 396–405 (2010).
Massaro, A. N. et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 194, 67–75.e61 (2018).
Chalak, L. F. et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J. Pediatr. 164, 468–474.e461 (2014).
Ennen, C. S. et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol. 205, 251.e1–7 (2011).
Massaro, A. N. et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr. Crit. Care Med. 14, 310–317 (2013).
Looney, A. M., Ahearne, C., Boylan, G. B. & Murray, D. M. Glial fibrillary acidic protein is not an early marker of injury in perinatal asphyxia and hypoxic-ischemic encephalopathy. Front Neurol. 6, 264 (2015).
Shah, D. K. et al. Raised plasma neurofilament light protein levels are associated with abnormal MRI outcomes in newborns undergoing therapeutic hypothermia. Front Neurol. 9, 86 (2018).
Wu, H. et al. Sbdps and Tau proteins for diagnosis and hypothermia therapy in neonatal hypoxic ischemic encephalopathy. Exp. Ther. Med. 13, 225–229 (2017).
Badawi, N. et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 317, 1554–1558 (1998).
Nelson, K. B. et al. Antecedents of neonatal encephalopathy in the vermont oxford network encephalopathy registry. Pediatrics 130, 878–886 (2012).
Acknowledgements
We thank all families for participating in clinical research, which allows us to continue to improve care for neonates. We would like to acknowledge Janet Brady, the occupational therapist who completed the Bayley III exams. We thank Melissa Lamb, MS, of Lamb Consulting, LLC, for editing our final draft.
Funding
Department development funding.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: Z.Y., K.W., M.D.W.; acquisition of data: H.X., L.S., R.D.A., R.L.P., C.R., M.A., D.R.; data analysis and interpretation: Z.Y., M.D.W., L.S.; drafting the article: M.D.W., Z.Y., L.S.; final approval of the version to be published: Z.Y., K.W., M.D.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained within 72 h of birth from parents of all neonates enrolled.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, Z., Xu, H., Sura, L. et al. Combined GFAP, NFL, Tau, and UCH-L1 panel increases prediction of outcomes in neonatal encephalopathy. Pediatr Res 93, 1199–1207 (2023). https://doi.org/10.1038/s41390-022-01994-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01994-0
This article is cited by
-
Usefulness of serum neurofilament light in the assessment of neurologic outcome in the pediatric population: a systematic literature review
European Journal of Pediatrics (2023)