Abstract
Background
To investigate mechanisms of injury and recovery in neonatal encephalopathy (NE), we performed targeted metabolomic analysis of plasma using liquid chromatography with tandem mass spectrometry (LC/MS/MS) from healthy term neonates or neonates with NE.
Methods
Plasma samples from the NE (n = 45, day of life 0–1) or healthy neonatal (n = 30, ≥36 weeks gestation) cohorts had LC/MS/MS metabolomic profiling with a 193-plex targeted metabolite assay covering >366 metabolic pathways. Metabolite levels were compared to 2-year neurodevelopmental outcomes measured by the Bayley Scales of Infant and Toddler Development III (Bayley-III).
Results
Out of 193 metabolites, 57 met the pre-defined quality control criteria for analysis. Significant (after false discovery rate correction) KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways included aminoacyl-tRNA biosynthesis, arginine biosynthesis, and metabolism of multiple amino acids. Significant disease pathways included seizures. In regression models, histidine and C6 sugar amine were significantly associated with cognitive, motor, and language and betaine with cognitive and motor Bayley-III composite scores. The addition of histidine, C6 sugar amine, and betaine to a Sarnat score-based clinical regression model significantly improved model performance (Akaike information criterion and adjusted r2) for Bayley-III cognitive, motor, and language scores.
Conclusions
Plasma metabolites may help to predict neurological outcomes in neonatal brain injury and enhance current clinical predictors.
Impact
-
Plasma metabolites may help to predict neurological outcomes in NE and supplement current clinical predictors.
-
Current metabolomics research is limited in terms of clinical application and association with long-term outcomes.
-
Our study presents novel associations of plasma metabolites from the first 24 h of life and 2-year neurodevelopmental outcomes for infants with NE.
-
Our metabolomics discovery provides insight into possible disease mechanisms and methods to rescue and/or supplement metabolic pathways involved in NE.
-
Our metabolomics discovery of metabolic pathway supplementations and/or rescue mechanisms may serve as adjunctive therapies for NE.
Similar content being viewed by others
Introduction
Neonatal encephalopathy (NE) is a common syndrome, occurring in as many as 3 per 1000 live births.1 Neurological dysfunction during the first few days of life defines NE, including clinical features of difficulty initiating or maintaining respiration, altered consciousness, and seizures.2,3 Approximately 29% of neonates with NE experience unfavorable outcomes of neurological death and disability, even with appropriately implemented therapeutic hypothermia.4 The efficacy of therapeutic hypothermia depends on accurately identifying injury severity.5,6 Grading the severity at birth relies predominately on the subjective Sarnat score; however, the Sarnat score is relatively imprecise in discriminating moderate from severe NE. With the advent of more sophisticated technology, amplitude-integrated encephalopathy, head ultrasound (HUS), and magnetic resonance imaging (MRI) are clinical modalities used to identify abnormal cortical function and patterns of damage.7,8 However, MRI is not diagnostic until 24 h after the injury, and for neonates treated with TH, TH devices are not MRI compatible delaying MRI use.9,10,11 In addition, although HUS can be used sooner than MRI and requires less logistical movement for equipment, HUS has low sensitivity for neonatal brain injury diagnosis or severity.12 Additional diagnostic, prognostic, and therapeutic tools derived from plasma analysis could be improved with a better understanding of NE etiology and underlying mechanisms of brain injury and prognosis.
NE is a syndrome that does not specify etiology and this remains unknown in nearly half of NE cases.2,13 Nonetheless, common factors include maternal characteristics, perinatal infections, hypoxic–ischemic encephalopathy (HIE), and neonatal conditions, including metabolic disorders and neonatal stroke.2,13 HIE animal models demonstrated that NE can result from metabolomic energy failure, leading to neuronal cell death and inflammatory damage.14,15,16 Moreover, the predominant findings of metabolomics in both animal models and infants with NE focus on changes of energy metabolite concentrations, including increased lactate and accumulation of tricarboxylic acid cycle intermediates.17,18,19,20
Metabolomics provides a useful method to investigate not only a potential panel of metabolites for diagnosis, prognosis, and therapy but also allows for holistic investigation of pathways. Mechanistic insights obtained by metabolomics would provide valuable information to understand the underlying network of interactions in neonatal brain injury and, more importantly, pathways to recovery. From a clinical standpoint, markers may augment existing diagnostic strategies. However, current research relevant to clinical application is limited, and more studies are needed that can link metabolomics with clinical measures of severity, and short- and long-term outcomes related to the severity of the disease.17 As an accessible biofluid, plasma in NE is especially useful because brain-derived markers, even if not brain-specific, per se, are more easily attributed to NE than in other patient populations where confounding conditions may limit their interpretation. To better understand mechanisms of injury and recovery in NE, we performed targeted metabolomic analysis of plasma by liquid chromatography with tandem mass spectrometry (LC/MS/MS) from neonates with NE and healthy term neonates.
Methods
In this prospective cohort study of NE, we performed targeted LC/MS/MS plasma metabolomics of neonates with NE and a control cohort. The study received institutional review board approval at all hospitals, and signed informed consent was obtained from the parent of each participant. Johns Hopkins University IRB approved the use of the cohort in this study.
Control patient cohort
Healthy term neonates (≥36 weeks gestation) had plasma samples collected in heparinized tubes from National Maternity Hospital Dublin and Coombe Women and Infants University Hospital from 2016 to 2018. Heparinized samples were processed to plasma within 90 min of collection, aliquoted, and stored at −80 °C. The control cohort neonates all had normal deliveries, normal Apgar scores, and did not require neonatal intensive care unit admission. The samples were stored in the Trinity Translational Medicine Institute Biobank in Dublin, Ireland. De-identified plasma samples (n = 30) from neonatal controls were collected from day of life (DOL) 0 to 7 with a median collection on DOL 2 and were analyzed in collaboration with the Neonatal Inflammation and Multiorgan Dysfunction and Brain Injury Research group (NIMBUS) at Trinity College Dublin, Ireland (JHU MTA A33285). Protected health information was not exchanged between the European and American study teams, in compliance with European Union Human Subjects Research Policies.
NE patient cohort
Neonates with NE were defined as requiring resuscitation at birth and having an abnormal neurological examination. Inclusion criteria were as follows: all infants with NE grade II/III (Sarnat score)21 within the first 6 h of life requiring therapeutic hypothermia, NE in the first 48 h of life without therapeutic hypothermia, or postnatally diagnosed with brain injury on cranial ultrasound.22,23 Exclusion criteria consisted of maternal substance abuse and major congenital abnormalities. The NE cohort had plasma samples collected as described above. De-identified plasma samples from DOL 0 to 1 (n = 45) were stored in the Trinity Translational Medicine Institute Biobank in Dublin, Ireland.
Metabolomics
Plasma samples stored at −80 °C were shipped on dry ice to the Molecular Determinants Core at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida for metabolomic profiling using a targeted panel of 193 metabolites representing >366 metabolic pathways (Supplemental Table 1). MRM transitions and retention times for all 193 compounds in the method were determined using reference standards purchased from Sigma-Aldrich (St. Louis, MO) and Cayman Chemical Company (Ann Arbor, MI). A full list of transitions and methods are available via a material transfer agreement from Johns Hopkins School of Medicine (mta@jhu.edu—JHU reference number C16787).
For plasma sample preparation, 50 μl of the sample was mixed with 300 μl of ice-cold acetonitrile (Fisher Chemical Optima™ LC/MS, A955, Hampton, NH) and 50 μl of water (Fisher Chemical Optima™ LC/MS, W6), and filtered on Supelco 96-Well Protein Precipitation Filter Plate (Sigma-Aldrich, 55263-U—98539) using a PlatePrep 96-well Vacuum Manifold (Sigma-Aldrich, 57192-U). Ten microliters of each filtered sample was pooled together into a quality control (QC) pool to be run alongside the samples at regular intervals.
Samples (50 μl) or QC pool (100 μl) were injected in the mass spectrometer (Shimadzu 8060 triple quadrupole, Kyoto, Japan) with an electrospray ionization source used in both positive and negative mode with resolution set to 1 Da. Source conditions were a nebulizing gas flow at 3 L/min, heating gas flow set at 10 L/min, and interface temperature at 300 °C; desolvation line temperature was 250 °C, heat block temperature 400 °C, and drying gas flow at 10 L/min. Chromatography was performed with the following gradient: 0% mobile phase B from 0 to 2 min, then ramped to 24% mobile phase B from 2 to 5 min, then ramped to 34% mobile phase B from 5 to 11 min, and then ramped to 76% mobile phase B from 11 to 15 min, and finally equilibrated at 0% B for 2 min. Chromatography was performed on a pentafluorophenylpropyl (PFPP) 150 mm × 2.1 mm ID, 3 μM particle size column (Suppelco, Bellefonte, PA). Mobile phase A was water, 2% acetonitrile, and 0.1% formic acid and mobile phase B was acetonitrile with 2% water and 0.1% formic acid.
Each batch of samples was run with a System Suitability QC sample, which was created from commercially available plasma, and extracted in our laboratory using the sample preparation method described above. The purpose of this QC sample was to act as a purely analytical control that assures proper operation of the entire LC-MS instrumentation from the HPLC components to the mass spectrometer. The result of this QC sample was determined by the response of known metabolites (asparagine, methionine, and tryptophan) in the QC sample for retention time, raw signal, and signal to noise. Sample batches that passed QC analysis were then used in analyses.
Data analysis
Descriptive statistics of the cohort are presented as the median and interquartile range (IQR) or percentages, as appropriate. For the descriptive statistics, Mann–Whitney U tests were used to account for deviations from a normal distribution in the cohorts and Fisher’s exact test was used to account for the small sample sizes. For univariable analysis, unpaired t tests were used to compare metabolite levels differentially expressed between the control and NE cohort and outcomes within the NE cohort, using a false discovery rate (FDR) of 5% (Benjamini–Hochberg correction). In order to determine the association between metabolites and patient outcomes, metabolites were normalized (scaled and centered).
Logistic regression models for binary outcomes and linear regression models for continuous outcomes used general estimating equations. Given the small sample size, all models were adjusted for sex, gestational age, and Sarnat score. Missing values in clinical characteristics were generally rare and without an obvious pattern; if necessary, mean imputation was used to replace missing values. The small number of missing values did not justify the use of a more complex imputation method in this case. For each model, stepwise selection was performed (p ≤ 0.05 to enter, p > 0.05 to remove).
Neurodevelopmental outcomes related to NE were evaluated using the cognitive, motor, and language composite scores of the Bayley Scales of Infant and Toddler Development III (Bayley-III) at 2 years.24 The Bayley-III was evaluated as a continuous and binary variable. For binary analysis, we stratified into two groups (1) normal, scores ≥100, (2) abnormal, scores <100 and death, setting a low threshold to increase the sensitivity in this discovery study.25 The patients who died prior to neurodevelopmental follow-up were assigned a score of 39 for the continuous Bayley-III analysis.26
The samples were all run within the same batch, and as such, no batch effect/batch correction was included. To evaluate the intra-batch effect, we utilized the CVs of the QC pool, which was run at a regular interval during the analysis. Missing values in metabolite levels were fairly frequent and were replaced by half the minimum detectable level value. Given the small sample size, no attempt at cross-validation, bootstrapping or the creation of a predictive model was performed. Statistical analysis was performed using R version 3.6.2.27 We utilized MetaboAnalyst28 to investigate the top differentiating metabolites from the control vs NE univariable analysis and associations with disease pathways.
Results
Cohort demographics
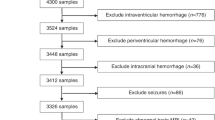
There were 30 neonates in the healthy control cohort and 45 neonates in the NE cohort available for analysis. The descriptive statistics of the cohorts are included in Table 1 and the clinical outcomes are described in Table 2. The control cohort was slightly younger than the NE cohort (39.1 vs 40.7 weeks), had higher first blood gas pH (7.3 vs 7.0), and a higher 5-min Apgar score (10 vs 5), respectively. The NE cohort included predominately (78%) moderate-to-severe NE (Sarnat score 2–3) and 67% of the cohort had seizures. The majority of infants with NE had low Barkovich MRI scores of 0 for basal ganglia (87%), watershed (83%), and basal ganglia/watershed ratio (78%). At 2 years follow-up, the majority of infants with NE had normal Bayley-III (score ≥100) for cognitive (68%), motor (68%), and language (53%) composite scores.
Metabolites in the control and NE cohort
In the 193-plex targeted metabolite assay, 57 metabolites were detected and quantified from the plasma samples. The differences in metabolites between the control and NE cohort are reported in Table 3, with the associated volcano plot in Fig. 1. Twenty-four metabolites were significantly different between NE infants and controls (FDR-adjusted p values <0.05, Table 3). All were increased in infants with NE compared to controls, except guanidinosuccinic acid, which was decreased in NE cases. The significant individual metabolites were evaluated using Metaboanalyst for disease and metabolic pathways.28 Significant associated KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways included alanine, aspartate, glutamate, cysteine, methionine, and histidine metabolism and arginine biosynthesis (Fig. 2). Using metabolite set enrichment analysis (MSEA; Fig. 3), plasma l-aspartic acid, l-alanine, l-asparagine, citrulline, l-methionine, l-phenylalanine, l-histidine were associated with neurologic disease pathways including acute seizures (FDR-adjusted p < 0.001), different seizure disorders (FDR-adjusted p < 0.001), and refractory localization-related epilepsy (FDR-adjusted p = 0.00192).29,30
The top metabolites from the univariable analysis of metabolites in control vs neonatal encephalopathy were investigated to find associated pathways. The top 5 associated disease pathways are shown here: aminoacyl-tRNA biosynthesis, alanine, aspartate, and glutamate metabolism, arginine biosynthesis, cysteine and methionine metabolism, and histidine metabolism.
The top metabolites from the univariable analysis of metabolites in control vs neonatal encephalopathy were investigated to find associations with disease. This included acute seizures (p < 0.001), different seizure disorders (p < 0.001), and refractory localization-related epilepsy (p = 0.00192) within the top 5 outcomes.
Metabolites and clinical outcomes
In univariable analysis, C6 sugar amine and citrulline were increased in neonates with moderate-to-severe encephalopathy (Sarnat 2–3, fold change (FC) 6.1, p < 0.001 and FC 1.7, p < 0.001, respectively) and C6 sugar amine were increased in neonates with seizures (FC 6.6, p < 0.001). Methyladenosine was increased in infants with a decreased Bayley-III language composite scores (FC 1.3, p = 0.001). No metabolites were significantly associated with a linear change in 5-min Apgar or a first blood draw pH.
Multivariable regression models adjusted for gestational age, sex, and Sarnat score were created to determine the association of metabolites with Bayley-III composite score outcomes (Table 4). Of the metabolites quantified, only C6 sugar amine, histidine, and betaine were significantly associated with cognitive and motor and C6 sugar amine and histidine with language outcomes. To determine if metabolites could improve the associations of clinical variables with Bayley-III composite score outcomes, we developed a base clinical model including Sarnat score, gestational age, and sex as a benchmark. We then explored if adding the significant metabolites identified in linear regression modeling (C6 sugar amine, histidine, and betaine) could improve the fit of the clinical model. The Sarnat-based clinical model was only marginally associated (p = 0.04) with Bayley-III Cognitive composite scores only. When the significant metabolites (C6 sugar amine, histidine, and betaine) were added to the base clinical model, the metabolites significantly improved model fit for all three Bayley-III composite score outcomes (Table 5).
As not all the NE cases had therapeutic hypothermia, we performed sensitivity testing comparing metabolite ratios in NE neonates receiving therapeutic hypothermia vs not. There were no significant differences in this comparison (Supplemental Table 2).
Discussion
In this prospective cohort study, our targeted metabolomics discovery revealed metabolite accumulation may reflect metabolic pathways that are involved in predominate mechanisms of injury and repair in neonatal brain injury. Moreover, regression models using metabolic factors from plasma collected on DOL 0–1 improved clinical base models for associations with neurodevelopmental outcomes at 2 years. This highlights the potential clinical implementation of peripherally monitoring metabolism relevant to brain injury and the ability to diagnose, prognose, and monitor therapeutic efficacy in NE. Previous metabolomics studies using plasma and urine specimens have identified similar associations of arginine, alanine, aspartate, glutamate, and phenylalanine pathways with NE.19,20
We identified three metabolites (C6 sugar amine, histidine, and betaine) associated with worse neurodevelopmental outcomes in adjusted multivariable regression modeling. Note that the C6 sugar amine was developed using mannosamine as an analytical standard; however, due to limitations of the PFPP analytical method, we cannot differentiate between mannosamine and other C6 sugar amines. Signal differences could be due to one or more overlapping signals of similar compounds. Nonetheless, one C6 sugar amine of interest is mannosamine, a neuraminic acid component, playing an important role as a sialic acid precursor. Sialic acids are essential components for many cellular functions and alterations in its metabolic pathway, including mannosamine, have been shown to affect the brain, skeletal, and muscle development.30,31,32 Van Karnebeek et al. showed in the setting of mutations in the gene encoding the sialic acid synthase, mannosamine was elevated in patients with infantile-onset severe developmental delay, a developmental feature shared by infants with NE.31 Furthermore, mannosamine also serves another crucial function as a potent inhibitor of glycosylphosphatidylinositol (GPI)-anchored protein synthesis.33 Critically, the folate receptor is a GPI-anchored protein and is critically important in brain development. Taken collectively, if mannosamine is elevated and inhibiting folate receptor synthesis, then NE is a state with an extreme risk of folate system failure. Further refinement of our assays to resolve C6 sugar amines is required.
Histidine is an essential amino acid and has unique properties that allow it to play important cytoprotective roles, including proton buffering, metal ion chelation, and antioxidant interactions.34 Histidinemia is typically asymptomatic and considered to be a normal metabolic variant; however, when combined with a perinatal event, it has been associated with an increased risk for developing intellectual disability, behavioral problems, or learning disorders.35,36 Specifically, Ishikawa et al. showed that histidinemia was related to language disorders.35 Not only did our study show plasma histidine was higher in neonates with NE compared with healthy control neonates but also found histidine was higher in neonates with abnormal Bayley-III language composite scores. Histidine was also a significant parameter in our linear regression models for worse Bayley-III cognitive, motor, and language composite scores. In adults, histidine is reduced in patients with acute seizures and juvenile myoclonal epilepsy when compared to a control cohort.29 These discrepancies possibly demonstrate a dynamic histidine metabolism involved in a brain injury that may differ between the pediatric and adult populations. Collectively, histidinemia may represent an increased cytoprotective response to neonatal brain injury.
Betaine is a metabolite with a vital role as a methyl group donor in one-carbon metabolism, which is important for both cell nutrient status and detoxification.37,38 Betaine is also an osmoprotectant and has been shown to have anti-inflammatory effects, including inhibition of NLRP3 inflammasome activation.38 Betaine metabolism was shown to be impacted in traumatic brain injury in animal models39 and identified as a potential biomarker of perinatal asphyxia in human studies, as well.40,41 In our study, unlike C6 amine sugars and histidine, betaine showed a nonsignificant absolute decrease in the NE cohort compared to controls, but was a significant parameter in the linear regression models of Bayley-III cognitive and motor scores. Decreased betaine may play a mechanistic role in brain inflammation and edema in NE. Decreased levels of betaine also point to overall disruptions in metabolism, as betaine is dependent on choline availability. Choline is produced by glycerophospholipid metabolism.42 In fact, elevated levels of sn-glycero-3-phosphocholine suggest that phosphohydrolase activity is decreased in infants with NE, thus limiting the choline essential for betaine production. As choline is indicated as a parental nutrition supplement, it is possible that choline supplementation may be beneficial to infants with NE.43 In a small cohort of ten infants with HIE, Locci et al. showed relatively high levels of betaine in urine specimens compared to their control group, which decreased over time. The differences between our studies may be due to specimen source, sample size, and warrant additional study into this metabolite.44
Seizures are very common in NE and a high seizure burden has been associated with abnormal outcomes.45,46,47 Our MSEA analysis identified multiple amino acids previously associated with seizures.29,30 In addition, excitatory amino acids such as aspartic acid and glutamine were elevated in infants with NE compared to the control cohort. Excitatory amino acids have been shown to be elevated in humans after seizures.29 Evidence suggests that increased levels are involved in pathomechanisms of seizures resulting from hyperexcitability. In our study, it was not possible to determine the temporality of the blood sampling and seizure occurrence, but the data does suggest a risk for hyperexcitability. Future studies of longitudinal samples used to determine metabolite levels could reveal a threshold for predicting seizure development and possibly triaging infants to antiepileptic therapy.
While the focus of the manuscript is on the association with neurodevelopmental outcomes, the differences in the levels of static metabolites in the plasma may provide us with important mechanistic insights into the challenges associated with NE. Notably, we observed increased levels of circulating kynurenine and kynurenic acid. Kynurenine pathway activation can occur in both the periphery and in the brain.48 In the periphery, this pathway is activated in febrile illness as part of acute-phase response. In the case of NE, increased levels of kynurenine could also be a result of activation in the central nervous system by microglial cells related to the inflammatory process.49 Further, kynurenic acid is a strong inducer of the aryl hydrocarbon receptor, which has recently been shown to promote astrogliosis and neurogenesis in the rat brain.50 Collectively, with the increased levels of kynurenic acid/tryptophan in the NE cohort, we hypothesize that circulating kynurenic acid is associated with neuroinflammation and neuroprotection.51,52
Limitations of this study include a small sample size, which precluded any cross-validation, bootstrapping, or creation of predictive models. Therefore, all associations identified in this study should be treated as hypothesis-generating and unconfirmed. Additional limitations include sample variation over 24 h and heterogeneity of NE cases. The criteria for inclusion included neonates with NE identified within 6 h with therapeutic hypothermia, identified within 48 h without therapeutic hypothermia, and postnatally with brain injury on cranial ultrasound. Although this may reflect heterogeneity in cases, all neonates included had perinatal asphyxia. Furthermore, we investigated if there were any significant differences between the metabolite results of the NE infants who received or did not receive therapeutic hypothermia and found no significant differences. We used a low threshold for determining an abnormal Bayley-III score (<100) to facilitate discovery. Therefore, our discovery of C6 sugar amine, histidine, and betaine with adverse outcomes will need to be validated with a larger cohort. Finally, our chromatography method could not distinguish between C6 amine sugars with the same peak profile and limits this study’s ability to identify the specific C6 sugar amine associated with the mechanistic or clinical features of NE. Future studies will include alternative methods to differentiate between C6 sugar amines for clarity.
Conclusion
Our metabolomics discovery using plasma samples in the first 24 h of life provided novel insight into the underlying network of metabolic interactions in neonatal brain injury, and moreover, potential pathways for recovery. Histidine, C6 sugar amine, and betaine were associated with Bayley-III composite scores at 2 years after birth, suggesting that their respective metabolic pathways could be involved in brain repair/injury. Methods to rescue and/or supplement these metabolic pathways may serve as adjunctive therapies to therapeutic hypothermia. Larger validation studies are warranted to further investigate the relationship between these metabolic pathways and neonatal brain injury before clinical implementation.
References
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Molloy, E. J. & Bearer, C. Neonatal encephalopathy versus hypoxic-ischemic encephalopathy. Pediatr. Res. 84, 574 (2018).
Volpe, J. J. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol. 72, 156–166 (2012).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy a randomized clinical trial. JAMA 318, 57–67 (2017).
Wyatt, J. S. et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 119, 912–921 (2007).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Ferriero, D. M. Neonatal brain injury. N. Engl. J. Med. 352, 839–839 (2005).
Gerner, G. J. et al. Transfontanellar duplex brain ultrasonography resistive indices as a prognostic tool in neonatal hypoxic-ischemic encephalopathy before and after treatment with therapeutic hypothermia. J. Perinatol. 36, 202–206 (2016).
American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Neonatal encephalopathy and neurologic outcome, second edition. Obstet. Gynecol. 123, 896–901 (2014).
Barkovich, A. J. et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. Am. J. Neuroradiol. 27, 533–547 (2006).
McKinstry, R. C. et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59, 824–833 (2002).
Graham, E. M., Everett, A. D., Delpech, J. C. & Northington, F. J. Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr. Opin. Pediatr. 30, 199–203 (2018).
Nelson, K. et al. Antecedents of neonatal encephalopathy in the Vermont Oxford Network Encephalopathy Registry. Pediatrics 130, 878–886 (2012).
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Wagner, S. R. & Lanier, W. L. Metabolism of glucose, glycogen, and high-energy phosphates during complete cerebral ischemia: a comparison of normoglycemic, chronically hyperglycemic diabetic, and acutely hyperglycemic nondiabetic rats. Anesthesiology 81, 1516–1526 (1994).
Smith, M. L., Von Hanwehr, R. & Siesjo, B. K. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J. Cereb. Blood Flow. Metab. 6, 574–583 (1986).
Efstathiou, N., Theodoridis, G. & Sarafidis, K. Understanding neonatal hypoxic-ischemic encephalopathy with metabolomics. Hippokratia 21, 115–123 (2017).
Sánchez-illana, Á. et al. Evolution of energy related metabolites in plasma from newborns with hypoxic-ischemic encephalopathy during hypothermia treatment. Sci. Rep. 7, 1–12 (2017).
Piñeiro-Ramos, J. D. et al. Metabolic phenotypes of hypoxic-ischemic encephalopathy with normal vs pathologic magnetic resonance imaging outcomes. Metabolites 10, 109 (2020).
Piñeiro-Ramos, J. D. et al. Noninvasive monitoring of evolving urinary metabolic patterns in neonatal encephalopathy. Pediatr. Res. https://doi.org/10.1038/s41390-021-01553-z, 1–8 (2021).
Sarnat, H. B. & Sarnat, M. S. Encephalopathy fetal distress: a clinical and and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Sweetman, D. et al. Coagulation profiles are associated with early clinical outcomes in neonatal encephalopathy. Front. Pediatr. 7, 1–7 (2019).
O’Hare, F. M. et al. Serial cytokine alterations and abnormal neuroimaging in newborn infants with encephalopathy. Acta Paediatr. Int. J. Paediatr. 106, 561–567 (2017).
Bayley, N. Bayley Scales of Infant and Toddler Development Manual 3rd edn (The Pscyhological Corporation, 2006).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Tobin, J. Estimation of relationships for limited dependent variables. Econometrica 26, 24–36 (1958).
R Core Team. R: A Language and Environment for Statistical Computing (R Core Team, 2019).
Pang, Z., Chong, J., Li, S. & Xia, J. Metaboanalystr 3.0: Toward an optimized workflow for global metabolomics. Metabolites 10, 186 (2020).
Rainesalo, S. et al. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem. Res. 29, 319–324 (2004).
Schnaar, R. L., Gerardy-Schahn, R. & Hildebrandt, H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518 (2014).
van Karnebeek, C. D. M. et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 48, 777–784 (2016).
Wen, X. Y. et al. Sialic acid catabolism by N-acetylneuraminate pyruvate lyase is essential for muscle function. JCI insight 3, e122373 (2018).
Lisanti, M. P., Field, M. C., Caras, I. W., Menon, A. K. & Rodriguez-Boulan, E. Mannosamine, a novel inhibitor of glycosyl-phosphatidylinositole incorporation into proteins. EMBO J. 10, 1969–1977 (1991).
Holecek, M. Histidine in health and disease: metabolism, physiological importance, and use as a supplement. Nutrients 12, 1–20 (2020).
Ishikawa, M. Developmental disorders in histidinemia - follow-up study of language development in histidinemia. Acta Paediatr. Jpn. 29, 224–228 (1987).
Scriver, C. R. & Levy, H. L. Histidinaemia. Part I: reconciling retrospective and prospective findings. J. Inherit. Metab. Dis. 6, 51–53 (1983).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Zhao, G. et al. Betaine in inflammation: mechanistic aspects and applications. Front. Immunol. 9, 1–13 (2018).
Chitturi, J., Li, Y., Santhakumar, V. & Kannurpatti, S. S. Early behavioral and metabolomic change after mild to moderate traumatic brain injury in the developing brain. Neurochem. Int. 120, 75–86 (2018).
Locci, E. et al. Exploring perinatal asphyxia by metabolomics. Metabolites 10, 1–19 (2020).
Sánchez-illana, Á., Piñeiro-ramos, J. D. & Kuligowski, J. Seminars in fetal and neonatal medicine small molecule biomarkers for neonatal hypoxic ischemic encephalopathy. Semin. Fetal Neonatal Med. 25, 101084 (2020).
Laboratories, K. Glycine, serine and threonine metabolism - reference pathway. KEGG Pathway https://www.genome.jp/pathway/map00260 (2019).
Buchman, A. L. The addition of choline to parenteral nutrition. Gastroenterology 137, S119–S128 (2009).
Locci, E. et al. A longitudinal 1 H-NMR metabolomics analysis of urine from newborns with hypoxic- ischemic encephalopathy undergoing hypothermia therapy. Clinical and medical legal insights. PLoS ONE 13, e0194267 (2018).
Andrade, E., Chavez, W., Shaikh, Z. I. & Torres, A. R. Neonatal encephalopathies: a clinical perspective. Cureus 11, e4948 (2019).
Kharoshankaya, L. et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic–ischemic encephalopathy. Dev. Med. Child Neurol. 58, 1242–1248 (2016).
Wusthoff, C. J. et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Child Neurol. 26, 724–728 (2011).
Badawy, A. A. B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1178646917691938 (2017).
Lovelace, M. D. et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 112, 373–388 (2017).
Chen, W. C. et al. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J. Neuroinflamm. 16, 1–13 (2019).
Sweetman, D. U. et al. Neonatal encephalopathy is associated with altered IL-8 and GM-CSF which correlates with outcomes. Front. Pediatr. 8, 1–7 (2021).
Zareen, Z. et al. Cytokine dysregulation persists in childhood post neonatal encephalopathy. BMC Neurol. 20, 1–9 (2020).
Acknowledgements
We thank the patients and their families for their participation and contributions to this study. We also thank the Everett research group at Johns Hopkins University School of Medicine for their support and contributions. We thank the Johns Hopkins University School of Medicine Scholarly Concentration mentor Dr. Meredith Atkinson and the Johns Hopkins University School of Medicine Dean’s Funding for their support and contributions.
Funding
This work was supported by NIH NICHD R01HD086058 (to A.D.E. and F.N.); Health Research Board, Ireland; Trinity College Dublin; National Children’s Research Centre.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: B.D.F., E.M., T.S., J.Z., M.S., V.D., D.S., L.K., M.O’D., A.R., R.H., G.E., C.M., D.G., F.N. and A.D.E. Drafting the article or revising it critically for important intellectual content: B.D.F., E.M., A.R., R.H., C.M., D.G., F.N. and A.D.E. Final approval of the version to be published: B.D.F., E.M., T.S., J.Z., M.S., V.D., D.S., L.K., M.O’D., A.R., R.H., G.E., C.M., D.G., F.N. and A.D.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Signed informed consent was obtained from the parent of each participant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Friedes, B.D., Molloy, E., Strickland, T. et al. Neonatal encephalopathy plasma metabolites are associated with neurodevelopmental outcomes. Pediatr Res 92, 466–473 (2022). https://doi.org/10.1038/s41390-021-01741-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01741-x