Abstract
Purpose
To explore the effectiveness of flexible bronchoscopy in pediatric Mycoplasma pneumoniae pneumonia (MPP).
Methods
This retrospective cohort study included children with MPP admitted between 2016 and 2019 in Shanghai. Tracheobronchial manifestations, etiologic findings, therapeutic effect, and health-economic indicators were assessed in bronchoscopy (plus bronchoalveolar lavage (BAL)) and non-bronchoscopy group. We used propensity-score matching and multivariable logistic regression to investigate the effect of bronchoscopy and BAL on disease recovery.
Results
In 900 children with MPP, 24/278 (8.6%) of those who underwent bronchoscopy had sputum plugs. Coinfection rate was four-fold enhanced by BAL (19.6% vs. 4.5%, p < 0.01) in patients with severe MPP (SMPP) and nearly doubled (10.8% vs. 5.9%, p = 0.03) in those without SMPP, compared with no BAL. Total of 224 (24.9%) patients had multilobar consolidation; after BAL, a significantly shorter lesion-resolution duration was observed on imaging (OR: 0.2, 95% CI: 0.0−0.7). However, longer fever duration (OR: 2.8, 95% CI: 1.7−4.8), hospital stay (OR: 3.1, 95% CI: 1.9−5.1), and higher costs were found in the bronchoscopy group than in the non-bronchoscopy group.
Conclusions
Through BAL, coinfection may explain one-fifth of causes for SMPP. Bronchoscopy with BAL may increase the detection rate of pathogen and resolve pulmonary lesions in patients with multilobar consolidation.
Impact
-
Flexible bronchoscopy with bronchoalveolar lavage is of great assistance in the timely detection of coinfection, sputum plug and inflammatory polyps in children with Mycoplasma pneumoniae pneumonia (MPP), and improves the recovery of lung damage in MPP patients with multilobar consolidation.
-
This study provides new insights into the indications of flexible bronchoscopy for the diagnosis and treatment of pediatric patients with MPP.
Similar content being viewed by others
Introduction
Mycoplasma pneumoniae is the leading cause of community-acquired pneumonia (CAP) in school-aged children and young adults.1,2 Approximately a quarter of patients with M. pneumoniae pneumonia (MPP) experience prolonged fever, worsening of clinical symptoms, and deteriorating radiological findings, despite appropriate macrolide therapy for 7 days or longer.3,4 Some even develop severe CAP or extrapulmonary manifestations, including pleural effusion, multilobar infiltrates, hypoxia,5 hemolysis, mucocutaneous disease, and central nervous system manifestations.6,7 Pathological studies focusing on severe M. pneumoniae pneumonia (SMPP) have been quite limited due to the rarely obtained specimens; subsequently, speculations continue on why a case of pneumonia progress to severe or critical stage. The presence of a resistant M. pneumoniae strain has been suggested as an important factor8; however, recent research has revealed that macrolide-resistant M. pneumoniae is not associated with the infection’s clinical severity, including radiographic findings, hospitalization rates, viral coinfections, mean duration for antimicrobial treatment, and clinical outcomes.9 Moreover, the proportion of MPP hospitalization requiring intensive care has no corresponding increase as macrolide resistance increases.10
Coinfections may also account for certain clinical manifestations attributed to SMPP; the emerging revolutionary technology is being intensively applied to pathogen identification. In young children with M. pneumoniae infections, the codetection rate of viruses by reverse transcription-polymerase chain reaction in the nasopharyngeal aspirate is as high as 30%.11 Due to the small fraction of high-quality sputum samples obtained from infected children, the coinfection rate of other bacteria with M. pneumoniae in lower respiratory tract remains under-investigated. Recent evidence supports the feasibility and safety of performing diagnostic flexible bronchoscopy (bronchoscopy) when it is difficult to collect sputum.12 Performing bronchoscopy would increase the sensitivity of bacterial detection in patients with SMPP through examination of bronchoalveolar lavage fluid (BALF), over that of blood and sputum, and also more clearly reveal pathological damage. In children with MPP, mucus accumulation in the airways and bronchial cast formation have been frequently observed13; however, studies specifically addressing these issues are lacking. In this study, we examined the effect of bronchoscopy on 900 hospitalized patients with MPP over a period of 3 years.
Materials and methods
Patients and study design
This was a cohort study of patients with MPP admitted to Xinhua Hospital, affiliated to Shanghai Jiao Tong University School of Medicine between February 2016 and July 2019. MPP was diagnosed based on the following conditions: (i) fever, cough, or auscultatory findings and a pulmonary infiltrate visible on chest imaging; and (ii) M. pneumoniae DNA detected in nasopharyngeal secretions by polymerase chain reaction (PCR), or ≥4-fold changes in M. pneumoniae IgM and IgG antibody titer between paired acute and convalescent sera, according to the Infectious Diseases Society of America guidelines of 2018.14,15 Patients with congenital heart diseases, motor developmental delay, and immunodeficiency were excluded. Informed consent was waived as the data were analyzed anonymously.
For every patient, blood samples were obtained for bacterial culture; respiratory syncytial virus, influenza A and B viruses, parainfluenza viruses, adenoviruses, Legionella pneumophila, Coxiella burnetii, Chlamydia pneumoniae, and M. pneumoniae serology for IgG and IgM antibodies was measured. Nasopharyngeal secretion samples were obtained for the detection of C. pneumoniae and M. pneumoniae using PCR. Conventional procedures for BALF microbiologic testing were performed in patients who underwent bronchoscopy, including cultures for bacteria, mycobacteria, and fungi.16 During this time, additional samples were sent to Huada Laboratories (Shenzhen, China) for next-generation sequencing (NGS), including sample processing and DNA extraction, construction of DNA libraries and sequencing, and bioinformatic analysis.17 Additional data are given in supplement material.
Bronchoalveolar lavage under bronchoscopy
After fasting for >6 h, patients were sedated with intravenous midazolam (0.1–0.2 mg/kg), and topical anesthesia with 1% lidocaine was applied to the nose, vocal cords, and trachea. Three types of flexible bronchoscopes were used; Olympus BFXP40, BF-3C30, and BF-P40 (Olympus Optical, Tokyo, Japan), depending on the patient’s age and body weight. The bronchoscope was wedged in the subsegmental bronchus of the most affected lobe seen on the chest radiograph. BAL with normal saline (weight < 20 kg: 1 mL/kg/time, 3 times; weight >20 kg: 20 mL/time, 3 times) was performed with −25 to −100 mmHg (1 mmHg = 0.133 kPa) suction, as per the Official American Thoracic Society Technical Standards.18 We also collected BALF samples for microbiological determinations. All manipulations were performed under sterile conditions. During the procedure, heart rate, respiratory rate, and saturation of pulse oxygen (SpO2) were monitored continuously. In case of occurrence of hypoxia, oxygen of appropriate concentration was administered immediately, and the procedure was stopped when necessary. The indications for bronchoscopy in our hospital included patients with recurrent/persistent atelectasis, recurrent pneumonia, poor response to standard anti-MP therapy treatment, suspected coinfection and severe conditions such as consolidation that requires rinsing and draining of infection.3,18,19,20,21,22
Data collection
We collected patient demographics (age, sex, weight, and parents’ income), clinical data (fever duration before admission, hypoxemia, neurological symptoms and signs (convulsion, seizures, lethargy, and abnormal reflex), and encephalitis), imaging features (atelectasis, pleural effusion, and lobar consolidation based on chest radiographs or low-dose computed tomography (CT) scans), and laboratory test results (interleukin (IL)-1, IL-2 receptor (IL-2R), IL-6, IL-8, IL-10, lactate dehydrogenase (LDH), C-reactive protein (CRP), partial pressure of CO2, white blood cell count, platelet count, total bilirubin, and creatinine) from medical histories. Low-dose CT was performed on a 64-detector row CT (Brilliance iCT, Philips) with 80–100 kV and 20–50 mA. The data were missing for IL (n = 253) and LDH (n = 125). Hypoxemia was defined as any recorded oxygen saturation <92% based on room-air pulse oximetry.23 The body temperature was monitored every day, and fever was defined as a body temperature exceeding 37.5 °C (99.5 °F). Routine blood and CRP examination were examined at admission and followed-up every 2−3 days until discharge. The data from the chest radiographs and CT scans during hospitalization were retrospectively collected.
After discharge, the patients were followed-up at 2 weeks, 1 month, and 3 months until a physician from the Infectious Diseases or Respiratory Department confirmed the resolution of pulmonary lesions on imaging. A re-examination of low-dose CT scan was considered in patients with pulmonary consolidation, atelectasis, pleural effusion and multiple lobes infiltration.
Each patient’s medical history was re-evaluated by a group of pediatricians, radiologists, and infection specialists. Severe pneumonia was assessed according to the guidelines by the Paediatric Infectious Diseases Society and Infectious Diseases Society of America on the management of CAP in infants and children older than 3 months.24 Refractory pneumonia was defined as prolonged fever, worsening of clinical symptoms, emergence of extrapulmonary complications, and deteriorating radiological findings, despite administration of appropriate macrolide therapy ≥7 days.3
Statistical analysis
All patients were classified into either the SMPP or the non-SMPP group, according to disease severity. Data are expressed as the median (25th−75th percentiles) and number (percentage) as appropriate. We used parametric (two-tailed t test) and nonparametric (Mann−Whitney U test) analyses for continuous data, as appropriate, and chi-squared (χ2) tests for categorical data to compare the two groups. First, patient characteristics between both groups were compared using descriptive statistics. Next, we summarized the bronchoscopic findings in the patients with and without SMPP. We subsequently analyzed the pathogen distribution in all patients. Finally, we assessed the effectiveness and cost of bronchoscopy in the patients with MPP using logistic regression analyses. Odds ratios (OR) were calculated for fever duration after admission, length of hospital stay, time to recovery of CRP, and resolution of pulmonary lesions on imaging, which represent the process of clinical improvement. The 75th-percentile value was used as the cut-off to calculate each OR.
To address indication bias, we applied 1:1 propensity-score matching according to age, income, fever duration before admission, duration of azithromycin therapy before admission, severe pneumonia, white blood cell count, and LDH, as these variables showed statistically significant differences between the bronchoscopy and non-bronchoscopy groups (excluding income, white blood cell count, LDH, and fever duration before admission). We also stratified the data by SMPP, lobar consolidation, and atelectasis to evaluate whether the effect was modified with the severity. Regarding confounding, we examined the collinearity of the variables in the model and discovered a strong collinearity between the following pairs of variables: IL-6 and IL-8, LDH and IL-2R, and LDH and IL-10. Finally, we adjusted the model for age, income, white blood count, LDH, fever duration before admission, and duration of azithromycin therapy before admission.
Statistical analyses were conducted using R (https://www.R-project.org, R foundation for Statistical Computing, Vienna, Austria). A two-tailed p value ≤0.05 was considered statistically significant.
Results
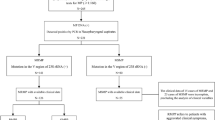
Among the 5112 children hospitalized due to CAP in our institution, 917 were diagnosed with MPP. Finally, the data of 900 patients with MPP were included for the analysis (Fig. 1). Most patients were pre-school and school-aged children (Table 1). A total of 224 (24.9%) patients had multilobar consolidation and 85 (9.4%) had atelectasis. The patients with SMPP were older, and with a higher rate of hypoxemia, neurological symptoms and signs, encephalitis, refractory pneumonia, atelectasis, pleural effusion and multilobar consolidation. Inflammation biomarkers such as IL-2 R, LDH, and CRP were significantly higher in patients with SMPP than those without SMPP. There were only seven intensive care unit admissions and no deaths.
There were 278 (30.9%) underwent bronchoscopy. Complications were quite mild, only one patient experienced decreased oxygen saturation (SpO2 < 85%), which resolved quickly upon ventilation with a bag-valve mask. No one had severe complications, such as pneumothorax, pulmonary hemorrhaging, or respiratory failure occurred during the procedure. A total of four (1.4%) patients had polyps and 24 (8.6%) had sputum plugs (Fig. 2). Notably, the proportion of patients with sputum plug was higher in the SMPP group than in the non-SMPP group (10.9% vs. 7.5%) although without significance.
Besides mycoplasma, other pathogens were detected in 74 (8.2%) of 900 patients. Viruses were the most frequent etiology (7.1%, 64/900), followed by bacteria (1.1%, 10/900) and fungi (0.1%, 1/900) (Table 2). Two viral coinfections in addition to M. pneumoniae were identified in three cases; one patient had fungi and virus coinfection. The overall rate of coinfection in the SMPP group (13.3%) was higher than that in the non-SMPP group (7.1%). Viruses and bacteria were more prevalent in the patients with SMPP; in particular, the detection rate of bacteria in the SMPP group was three times higher than that in the non-SMPP group (2.5% vs. 0.8%, p = 0.06). More coinfections were observed in the bronchoscopy group than in the non-bronchoscopy group, among the patients with SMPP (19.6% vs. 4.5%; p < 0.01) and without SMPP (10.8% vs. 5.9%; p = 0.03). In patients with SMPP, bacterial coinfection was only detected in those who underwent bronchoscopy. The rate of viral codetection in the SMPP bronchoscopy group was almost four times higher than that in the SMPP non-bronchoscopy group (15.2% vs. 4.5%, p = 0.03). Compared with patients without coinfections, those with coinfections were more likely to experience severe clinical symptoms (hypoxemia, 1.6% vs. 5.4%; severe pneumonia, 16.6% vs. 28.4%; refractory pneumonia, 28.1% vs. 52.7%, and multilobar consolidation, 23.6% vs. 39.2%) (Appendix Table 1). Patients with coinfections had a longer total fever duration and length of hospital stay than those without coinfections (median total fever duration: 9.5 [6.0−14.0] vs. 8.0 [6.0−10.0] days, p < 0.01; mean length of hospital stay: 9.3 ± 7.5 vs. 7.2 ± 3.6 days, p < 0.01).
Before propensity-score matching, there were differences between bronchoscopy and non-bronchoscopy group in the several of baseline variables. Patients who underwent bronchoscopy were older, heavier, and often severer with a higher rate of atelectasis and multilobar consolidation. The inflammation biomarkers such as IL-1 and IL-8 are slighter higher in the patients who did not undergo bronchoscopy, compared with those who did. After attempting to control for confounding factors especially illness severity using propensity-score matching, 474 (52.7%) patients remained for the final analysis, with appropriately balanced baseline characteristics (Appendix Table 2). The mean fever duration after admission (2.5 days vs. 1.7 days), hospital stay (9.0 days vs. 6.9 days) and the time to recovery of CRP (5.3 days vs. 4.7 days) were longer in the patients who underwent bronchoscopy than in those who did not; however, the time to resolution of pulmonary lesions on imaging was shorter in the bronchoscopy group (14.0 days) than in the non-bronchoscopy group (15.8 days). Further, undergoing bronchoscopy was associated with increased odds of experiencing fever for >3 days after admission (OR: 2.8, 95% confidence interval [CI]: 1.7−4.8), and a hospital stay >9 days (OR: 3.1, 95% CI: 1.9−5.1) (Table 3). Those who underwent bronchoscopy had an earlier resolution of pulmonary lesions on imaging, although the difference was not statistically significant. Bronchoscopy was not significantly associated with the recovery of CRP (OR: 1.5, 95% CI: 0.8−2.8).
In the matched cohort, 59/108 patients with SMPP underwent bronchoscopy. Undergoing bronchoscopy was associated with increased odds of staying in hospital for >13 days among the patients with SMPP, compared with that of patients with SMPP who did not undergo bronchoscopy (OR: 2.4, 95% CI: 1.1−5.1). Similarly, among the 118 patients with multilobar consolidation in the matched cohort, undergoing bronchoscopy increased the odds of a hospital stay for >9 days (OR: 3.3, 95% CI 1.3−8.5). No statistically significant ORs were observed among the 56 patients with atelectasis in the matched cohort. Undergoing bronchoscopy did not have a significant effect on the recovery of CRP in any subgroup. Notably, the time to resolution of pulmonary lesions on imaging was shorter in the bronchoscopy group than in the non-bronchoscopy group, in both the total matched cohort and in each of the three subgroups; although this difference was only statistically significant in patients with multilobar consolidation (OR for >19 days recovery time: 0.2, 95% CI: 0.0−0.7, p < 0.05). However, all patients eventually had complete resolution of pulmonary lesions, including patients in whom polyps were observed during bronchoscopy.
Table 4 summarizes the per capita medical costs of the bronchoscopy and non-bronchoscopy groups before and after propensity-score matching. The overall cost of healthcare per patient with MPP was $1335.1; the median cost of hospital charges for patients who underwent bronchoscopy was 66% higher than that for patients who did not undergo bronchoscopy ($1850.3 vs. $1114.1; p < 0.01). The direct operating costs increased by $137.3 due to the bronchoscopy procedure in the matched cohort. The patients who underwent bronchoscopy also incurred higher costs related to laboratory tests, imaging, and medication due to preoperative preparation, anesthesia, and post-surgical recovery (all p < 0.01 after propensity-score matching).
Discussion
In this large-scale clinical study of MPP in China, bronchoscopy was observed to be of great assistance in the timely detection of inflammatory polyps and coinfection. Bronchoscopy was capable of improving the detection rate of coinfection greatly, especially in the patients with SMPP, which contributed significantly to the definitive diagnosis and the cause of intractable condition. Regardless of whether the MPP is severe or not, BAL may improve the recovery from inflammatory multilobar consolidation; however, it does not help decrease the overall inflammatory reaction, and may even prolong fever duration and hospital stay. As such, bronchoscopy may not be suitable for therapeutic application in patients with MPP, unless it is suspected that SMPP requires further diagnosis. This study also provides evidence for the indication of bronchoscopy in CAP.
The role of obstructing lesion such as mucus plug in SMPP remains unclear. Mucus plug formation was commonly discovered under bronchoscopy in children with MPP13,25,26; however, few studies have emphasized its significance for the disease. A previous study found that mucus plug was significantly more commonly seen in the patients with refractory MPP than those with general MPP, and it combined with clinical features and laboratory data could improve the early identification of refractory pneumonia.27 In our study, the prevalence of sputum plugs in patients with SMPP was higher than in patients without SMPP, suggesting that sputum plugs may be associated with severe pneumonia. Severe bronchial inflammation and cilia abnormalities could make the airways produce extra mucus and decrease mucus clearance,28 leading to the formation of sputum plug. What’s more, we observed four cases of bronchial polyps under bronchoscopy, which are poorly recognized lesions in children with pneumonia.29,30
The etiologic diagnosis of CAP is known to be challenging in clinical practice. In several cases, the diagnosis is “non-resolving pneumonia”, which includes cases of presumed pneumonia that progress, resolve slowly, or fail to completely resolve despite the application of what is considered appropriate therapy. In a national survey, it has been found 27.7% of American patients with MPP had one or more concurrent viral infections9; however, this study does not link coinfection with clinical progress. In our study, upon BALF analysis, coinfection was observed in nearly one fifth of children with SMPP, especially bacterial infection. The detection rate of mucus plug and pathogen in the bronchoscopy and non-bronchoscopy groups may be associated with sampling, and it was not necessarily representative of the pathogens per se. However, in patients with the same severity, such as SMPP, we found that bronchoscopy significantly improved the detection rate of pathogens, with significant differences, which could address the clinical question. Thus, we inferred that coinfection may be a possible cause for SMPP, which may be overlooked as the sputum sample from the upper respiratory tract may be easily contaminated, and blood cultures/serological tests are of low specificity and sensitivity. BALF collected by bronchoscopy may be quite a suitable option for patients with pneumonia who do not respond to antibiotic treatment. Moreover, a major proportion of patients underwent bronchoscopy within 5 days of hospitalization. Thus, it is important to distinguish between initial coinfection and in-hospital infection after severe pneumonia. Furthermore, approximately 20% of presumed nonresponding CAP cases are due to non-infectious causes;31 to the best of our knowledge, the existence of non-infectious etiologies of pulmonary infiltrates in SMPP has not been studied. Atelectasis and pleural effusion are common abnormalities in children with MPP, at rates of 29% and 16%−26%, respectively.5,10,32 Since non-resolving pneumonia often develops as a result of poor cough or mucus plugging in the lower lobes, it may be warranted in patients with persistent atelectasis due to an unknown cause or suspected airway obstruction. Bronchoscopy is especially indicated for unresolving pneumonia complicated with atelectasis and a large area of consolidation,18,20 as it allows direct observation of the airways and acquiring samples directly from the infected lobe.
Bronchoscopy for therapeutic purpose remains controversial in children with CAP. Although it is of some benefits, bronchoscopy poses the risk of additional trauma to the airway and other complications. There are no absolute contraindications to performing BAL in patients with CAP and this may lead to overtreatment. In some places it is recommended in the guideline of refractory pneumonia.22 Bronchoscopy and BAL enable removal of mucous plugs or foreign bodies and may help relieve the immunological reaction by washing with warm saline.33 In a small-sample study (n = 46), BAL was substantially more effective than non-BAL in improving clinical symptoms and resolution of pulmonary lesions on radiography in children with SMPP.34 Bronchoscopy was also associated with shorter fever duration and improvement of pulmonary lesions in several studies.34,35 However, in these studies, the sample size is often limited with insufficient statistical power, and the definition of clinical prognosis were not defined standardly. In this study, we used the bronchoscopy to exclude malignant endobronchial obstruction in patents with MPP, remove any obstructing lesion especially mucus plug and the time of resolution of pulmonary lesions shortened after BAL, however, the fever time and length of hospital stay are extended. A possible explanation for this might be that flexible bronchoscope may spread the organisms form the localized lesion to normal areas of bystander lung by BAL, which leads to new infiltration or exacerbation.36 The longer recovery time may also be related to the mechanical damage, like alveolar flooding after BAL, postobstructive atelectasis from mucosal swelling, blood clots, or impaired mucociliary clearance caused by BAL. Furthermore, in our follow-up, the atelectasis resolved spontaneously under routine treatment without bronchoscopy, the same as those with polyps. More studies are needed in the therapeutical effect of BAL in patients with MPP.
There are several limitations to our study. First, as a retrospective single-centered study, selection bias may have been a possibility. To reduce confounding, we conducted propensity-score matching, after which the results coincided with those for the whole population. Second, the amount of BALF retrieved, the sampling conditions, and the time of transportation to the laboratory might have interfered with the positive culture rate of the samples. Thus, we strived to standardize every step of the procedure. Third, the NGS testing in our study was supported by our research funding, which will be a concern in clinical practice for most patients. In our study, all the BALF samples were transferred to a centralized laboratory for NGS testing, which could increase transportation costs and the turnaround time. For further studies, we are committed to exploring ways to reduce costs, including steaming workflow, automating library preparation, and localization of the sequencing platform.
Conclusions
Coinfection may be an important factor for SMPP, and may explain one fifth of the cause. Bronchoscopy is a good choice to confirm the diagnosis. Randomized clinical trials are warranted to evaluate the therapeutic application in patients with SMPP.
References
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845 (2015).
Morozumi, M., Takahashi, T. & Ubukata, K. Macrolide-resistant mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J. Infect. Chemother. 16, 78–86 (2010).
Tamura, A. et al. Methylprednisolone pulse therapy for refractory mycoplasma pneumoniae pneumonia in children. J. Infect. 57, 223–228 (2008).
Zhang, Y. et al. The clinical characteristics and predictors of refractory Mycoplasma Pneumoniae pneumonia in children. PLoS ONE 11, e0156465 (2016).
Kutty, P. K. et al. Mycoplasma Pneumoniae among children hospitalized with community-acquired pneumonia. Clin. Infect. Dis. 68, 5–12 (2019).
Al-Zaidy, S. A., MacGregor, D., Mahant, S., Richardson, S. E. & Bitnun, A. Neurological complications of Pcr-proven M. Pneumoniae infections in children: prodromal illness duration may reflect pathogenetic mechanism. Clin. Infect. Dis. 61, 1092–1098 (2015).
Olson, D. et al. Outbreak of Mycoplasma Pneumoniae-associated Stevens−Johnson syndrome. Pediatrics 136, e386–e394 (2015).
Yamazaki, T. & Kenri, T. Epidemiology of Mycoplasma Pneumoniae infections in Japan and therapeutic strategies for macrolide-resistant M. Pneumoniae. Front. Microbiol. 7, 693 (2016).
Waites, K. B. et al. Macrolide-resistant Mycoplasma Pneumoniae in the United States as determined from a National Surveillance Program. J. Clin. Microbiol. 57, e00968-19 (2019).
Lee, K. L. et al. Severe Mycoplasma Pneumoniae pneumonia requiring intensive care in children, 2010−2019. J. Formos. Med. Assoc. 120, 281–291 (2021).
Han, M. S. et al. Contribution of co-detected respiratory viruses and patient age to the clinical manifestations of Mycoplasma Pneumoniae pneumonia in children. Pediatr. Infect. Dis. J. 37, 531–536 (2018).
Loens, K., Van Heirstraeten, L., Malhotra-Kumar, S., Goossens, H. & Ieven, M. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. J. Clin. Microbiol. 47, 21–31 (2009).
Huang, L. et al. Independent predictors for longer radiographic resolution in patients with refractory Mycoplasma Pneumoniae pneumonia: a prospective cohort study. BMJ Open 8, e023719 (2018).
Miller, J. M. et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 67, e1–e94 (2018).
Xu, X. F., Li, X. J., Liu, J. L., Wu, L. & Chen, Z. M. Serum cytokine profile contributes to discriminating M. Pneumoniae pneumonia in children. Cytokine 86, 73–78 (2016).
Murdoch, D. R., O’Brien, K. L., Driscoll, A. J., Karron, R. A. & Bhat, N. Laboratory methods for determining pneumonia etiology in children. Clin. Infect. Dis. 54, S146–S152 (2012).
Li, Y. et al. Application of metagenomic next-generation sequencing for bronchoalveolar lavage diagnostics in critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 39, 369–374 (2019).
Faro, A. et al. Official American Thoracic Society Technical Standards: flexible airway endoscopy in children. Am. J. Respir. Crit. Care Med. 191, 1066–1080 (2015).
Nie, X., Cai, G. & Li, Q. Bronchoscopy in China: the Chinese Society of Respiratory Diseases Survey. Chest 136, 1186–1187 (2009).
Midulla, F. et al. Flexible endoscopy of paediatric airways. Eur. Respir. J. 22, 698–708 (2003).
Andrés-Martín, A. et al. Consensus document on community-acquired pneumonia in children. Senp-Separ-Seip. Archivos de. Bronconeumol.ía (Engl. Ed.) 56, 725–741 (2020).
National Health Commission of the People’s Republic of China, State Administration of Traditional Chinese Medcine. Guideline for diagnosis and treatment of community-acquired pneumonia in Children (2019 version). Chin. J. Clin. Infec. Dis. 12, 6–13 (2019).
Harris, M. et al. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Children: update 2011. Thorax 66(Suppl 2), ii1–ii23 (2011).
Bradley, J. S. et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76 (2011).
Xu, Q. et al. Prediction of bronchial mucus plugs formation in patients with refractory Mycoplasma Pneumoniae pneumonia. J. Trop. Pediatr. 63, 148–154 (2017).
Yan, Y., Wei, Y., Jiang, W. & Hao, C. The clinical characteristics of corticosteroid-resistant refractory Mycoplasma Pneumoniae pneumonia in children. Sci. Rep. 6, 39929 (2016).
Wang, L. et al. The early examination of combined serum and imaging data under flexible fiberoptic bronchoscopy as a novel predictor for refractory Mycoplasma Pneumoniae pneumonia diagnosis. Medicine 96, e9364 (2017).
Prince, O. A., Krunkosky, T. M., Sheppard, E. S. & Krause, D. C. Modelling persistent Mycoplasma Pneumoniae infection of human airway epithelium. Cell Microbiol. 20, 1066–1080 (2018).
Dincer, I., Demir, A., Akin, H., Melek, H. & Altin, S. A giant endobronchial inflammatory polyp. Ann. Thorac. Surg. 80, 2353–2356 (2005).
McShane, D. et al. Inflammatory endobronchial polyps in childhood: clinical spectrum and possible link to mechanical ventilation. Pediatr. Pulmonol. 34, 79–84 (2002).
Arancibia, F. et al. Antimicrobial treatment failures in patients with community-acquired pneumonia: causes and prognostic implications. Am. J. Respir. Crit. Care Med. 162, 154–160 (2000).
John, S. D., Ramanathan, J. & Swischuk, L. E. Spectrum of clinical and radiographic findings in pediatric Mycoplasma Pneumonia. Radiographics 21, 121–131 (2001).
Kapur, N. et al. Therapeutic bronchoscopy in a child with sand aspiration and respiratory failure from near drowning—case report and literature review. Pediatr. Pulmonol. 44, 1043–1047 (2009).
Wu, W. Z. & Liu, D. C. Observation of the efficacy of bronchoscopic alveolar lavage for severe Mycoplasmal Pneumonia in children and the changes in lung function. J. Pract. Med. 35, 132–135 (2019).
Shao, M. K., Zhou, Y., Du, K., Zhang, Y. & Deng, L. Treatment of children with severe Mycoplasma Pneumonia by electronic bronchoscope. Chin. J. Nosocomiol. 26, 4735–4737 (2016).
Mehta, A. C. & Minai, O. A. Infection control in the bronchoscopy suite. A review. Clin. Chest Med. 20, 19–32, ix (1999).
Acknowledgements
We thank all participants and staff of this study and the physicians at the Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine. This work was supported by the National Natural Science Foundation of China under grant numbers 81874265 and 82073561; Research Physician of the Peak Plateau Project of Shanghai Municipal Education Commission under grant number 2020002; Shanghai Science and Technology Commission under grant numbers 18411966600 and 19410740800; National Respiratory Field Key Laboratory Emergency Project numbers EKPG21-08 and National Ministry of Science and Technology-National Key R&D Program Project numbers 2021YFE0201900.
Funding
This work was supported by the National Natural Science Foundation of China under grant numbers 81874265 and 82073561; Research Physician of the Peak Plateau Project of Shanghai Municipal Education Commission under grant number 2020002; Shanghai Science and Technology Commission under grant numbers 18411966600 and 19410740800; National Respiratory Field Key Laboratory Emergency Project numbers EKPG21-08 and National Ministry of Science and Technology-National Key R&D Program Project numbers 2021YFE0201900.
Author information
Authors and Affiliations
Contributions
L.W. and Q.X. conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. S.X., H.L., L.Z. and J.A. collected data, carried out the initial analyses, and reviewed and revised the manuscript. Q.L., C.C., and X.Z. coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. L.H. and W.Z. designed the study and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for the content of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ethics Committee of Xinhua Hospital (XHEC-C-2018-107). This study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, L., Xie, Q., Xu, S. et al. The role of flexible bronchoscopy in children with Mycoplasma pneumoniae pneumonia. Pediatr Res 93, 198–206 (2023). https://doi.org/10.1038/s41390-021-01874-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01874-z
This article is cited by
-
Early predictors of delayed radiographic resolution of lobar pneumonia caused by Mycoplasma pneumoniae in children: a retrospective study in China
BMC Infectious Diseases (2024)
-
Comparison of the clinical characteristics in parents and their children in a series of family clustered Mycoplasma pneumoniae infections
BMC Pulmonary Medicine (2024)
-
Changes in coagulation markers in children with Mycoplasma pneumoniae pneumonia and their predictive value for Mycoplasma severity
Italian Journal of Pediatrics (2023)
-
Risk prediction model for long-term atelectasis in children with pneumonia
BMC Pulmonary Medicine (2023)
-
Optimization strategy for the early timing of bronchoalveolar lavage treatment for children with severe mycoplasma pneumoniae pneumonia
BMC Infectious Diseases (2023)