Abstract
Background
Kernicterus Spectrum Disorders (KSDs) result from hyperbilirubinemia-induced brain injury. We developed a Toolkit (KSD-TK) to predict the likelihood of KSDs. This study aims to validate the KSD-TK by comparing it to clinical diagnoses made by the Kernicterus Clinic in the Division of Neurology.
Methods
Through retrospective chart review, we completed a KSD-TK for 37 patients evaluated between 2011 and 2019 using highest bilirubin, newborn risk factors, neonatal exam, follow-up exam, auditory testing, tooth enamel, and MRI brain results. KSD-TK results were compared to the clinical diagnoses given by a kernicterus expert (SS).
Results
Of 37 patients, 29 were clinically diagnosed with kernicterus, including 14/14 with KSD-TK scored as “definite”, 14/15 “probable”, and 1/2 with “possible” kernicterus. None of 6 patients with KSD-TK “not kernicterus” were clinically diagnosed with kernicterus. Combining KSD-TK “definite” and “probable”, the KSD-TK has 96.6% sensitivity and 87.5% specificity. Each KSD-TK component had high sensitivity, but only three had specificity ≥0.75: auditory neuropathy spectrum disorder, abnormal movements and/or tone on follow-up exam, and abnormal globus pallidus and/or subthalamic nucleus on MRI.
Conclusion
The KSD-TK is a promising screening tool for patients at risk for kernicterus.
Impact
-
This study provides validation of a Kernicterus Spectrum Disorders (KSDs) Toolkit.
-
The toolkit provides screening criteria for predicting KSD diagnosis.
-
Scores of definite or probable have high sensitivity and specificity for KSDs.
-
Abnormal auditory processing, exam, and MRI were most specific for KSDs.
Similar content being viewed by others
Objective/background
Neonatal hyperbilirubinemia can result in Kernicterus Spectrum Disorders (KSDs) due to excess free unconjugated bilirubin crossing the blood−brain barrier, irreversibly damaging the basal ganglia, hippocampus, cerebellum, and cranial nerve nuclei, specifically the oculomotor, vestibular, and cochlear nuclei. This subsequently causes devastating motor and/or auditory dysfunction.1 Although it is a relatively rare disorder, kernicterus remains a major problem and cause of disability worldwide.2,3,4 Even in developed countries with neonatal surveillance and prevention programs, the incidence of kernicterus is estimated to be 1 per 44,000 live births in Canada from January 1, 2007 to December 31, 2008,5 which predicted 97 new cases per year in the USA at that time,6 and 82 per year currently based on a 16.5% decline in the USA annual birth rate from 2007 to 2020 (https://www.cdc.gov/nchs/data/vsrr/vsrr012–508.pdf). Children with kernicterus are cognitively normal but are afflicted with a lifetime of disability, including severe and often painful dystonia leading to difficulty with voluntary movements, and/or auditory neuropathy spectrum disorders with or without hearing loss.7 Parents of children with undiagnosed neurodevelopmental disorders and elevated bilirubin levels or jaundice in the neonatal period may question the possibility that their child could have a KSD.
Due to the lack of knowledge about KSDs as well as limited follow-up in the neonatal period, especially in resource-poor settings, many local physicians do not have the expertise they need to diagnose KSDs.8 The current practice to establish a kernicterus diagnosis is through clinical diagnosis by an expert familiar with the diagnosis, but physicians who specialize in KSDs are not always available.9 This has led to underdiagnosis and undertreatment of KSDs.10 While the Bilirubin-Induced Neurologic Dysfunction (BIND) scale can be used to determine the severity and progression of neonatal acute bilirubin encephalopathy, there are unfortunately no standardized diagnostic criteria to screen patients for kernicterus.11,12 In order to address this problem in our area, we created a Kernicterus Clinic at Children’s Mercy Hospital in 2011, which serves as a point of contact for clinicians or families to get more information on kernicterus and potentially come for a complete evaluation. This clinic is staffed by neurologists with experience in diagnosing and managing children with kernicterus, with multidisciplinary involvement from audiology, radiology, and other subspecialty services as needed, with a nursing coordinator to oversee patient care. The Kernicterus Clinic has been an excellent resource for some families, but we recognize that not all children can be evaluated in such a clinic.
In order to help parents and physicians predict the likelihood of KSDs in a standardized format, we developed a screening tool: The Kernicterus Spectrum Disorders Diagnostic Toolkit (KSD-TK). This toolkit looks at the child’s risk factors for KSDs and most recent neurologic and auditory evaluation in order to calculate the likelihood of KSDs. The toolkit was created using the risk factors determined by our kernicterus experts that contribute most to the clinical diagnosis of kernicterus. Numerical values were assigned to each of these risk factors to create a rating scale that estimates the likelihood of a kernicterus diagnosis. This toolkit could then be used by clinicians or potentially family members less familiar with kernicterus to estimate the likelihood of a kernicterus diagnosis in their patient or child. While not substituting for a clinical evaluation with a kernicterus expert, this tool would allow clinicians and families to estimate the likelihood of a KSD in their child and use this to determine if further evaluation is needed. The primary objective of this study is to estimate the accuracy of the KSD-TK by comparing the results of the KSD-TK to the clinical diagnosis by a kernicterus expert. The secondary objective is to learn which risk factors on the KSD-TK have the greatest association with a KSD diagnosis.
Methods
A retrospective chart review was performed to determine the validity of the KSD-TK result when compared to the clinical diagnosis given by a kernicterus expert (S.M.S.) at the Children’s Mercy Kansas City’s Kernicterus Clinic. Patient consent was not obtained for this study as it is a retrospective chart review, and no identifying information was included in the manuscript. The study was deemed exempt and granted a waiver of HIPAA Authorization under 45 CFR 164.512(i)(2)(ii) by the Institutional Review Board of Children’s Mercy Hospital.
Chart review and completion of the KSD-TK were performed by a project member (V.R.D.) who was not involved in the clinical diagnosis of the patients. As the information for the KSD-TK was obtained through viewing chart records from the patient’s visit to the Kernicterus Center, it was not possible to fully blind the chart review to the patients’ clinical diagnoses. However, as the KSD-TK focused on recording objective measures and clinical findings that were already outlined in the chart, it was determined that this did not contribute to significant bias for a preliminary study. Inclusion criteria included: age < 18 years, evaluation for KSDs at the Kernicterus Clinic from 2011 to 2019, and the presence of all necessary information to complete the KSD-TK in the patient’s chart. A total of 55 patient charts were reviewed from the Kernicterus Clinic database, but only 37 patients were ultimately included in the final study group; the 18 patients were excluded due to inadequate information in their charts or lack of formal evaluation by a clinic physician. Thirteen of the excluded patients sent records to the Kernicterus Clinic but were never formally evaluated or diagnosed in the Kernicterus Clinic. The remaining five patients were seen in the clinic but did not have sufficient information in their records or testing to complete the toolkit. The demographic data for the 37 patients included in the final study group are shown in Table 1 Demographic data for the 18 excluded patients were not included in this paper as most of these patients were not evaluated at our Kernicterus Clinic, and thus key information is lacking, and we were unable to make a definitive clinical diagnosis.
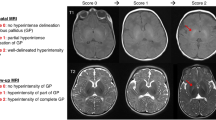
For each patient included, we completed a KSD-TK (Fig. 1) based on their highest bilirubin value, newborn risk factors, results of the neonatal exam, results of the last follow-up exam, presence or absence of dental enamel dysplasia in the deciduous (baby) teeth, auditory testing for Auditory Neuropathy Spectrum Disorder (ANSD), and presence or absence of abnormal hyperintensity of the global pallidus and/or the subthalamic nuclei on brain MRI to determine the KSD-TK diagnostic prediction. Infants with unerupted teeth were recorded as having normal enamel development for the purposes of this study. Given the known evolution of MR hyperintensities in kernicterus patients over time, a record of any MRI with this pattern of injury was considered in the results.13 Information about these qualitative factors were assigned quantitative values for each subdivision on the KSD-TK scoring sheet and then totaled to determine the final score (Fig. 1). Based on the total range of possible KSD-TK scores from 0 to 14 and a prior article on the diagnosis and treatment of KSDs,1 we classified the KSD-TK results as “definite” (score of 10–14), “probable” (score of 6–9), “possible” (score of 3–5), and “not kernicterus” (score of 0–2).
Each patient’s chart was reviewed and assigned a score for each question on this toolkit. The scores were totaled for each individual patient and used to determine the KSD-TK’s diagnostic prediction. Bilirubin levels are given in milligrams per deciliter (mg/dL) and micromoles per liter (μM), where 1 mg/dL bilirubin = 17.1 μM.
We first compared KSD-TK predictions to clinical diagnoses given after evaluation at our Kernicterus Clinic. The four prediction outcomes were also dichotomized (combining “definite” and “probable” kernicterus (scores 6–14) and combining “possible” and “not” kernicterus (scores 0–5)) to allow for estimation of the sensitivity and specificity of the KSD-TK in correctly predicting the clinical diagnosis. We then evaluated the KSD-TK predictions by logistic regression, using each individual risk factor (i.e., sub-scale score) as an explanatory variable one at a time, due to the small sample size and multicollinearity. The sensitivity, specificity, accuracy, and area under the curve of each individual risk factor were calculated as well to determine the utility of each risk factor included on the KSD-TK.
Results
After performing the chart review, we found that 29 of our 37 patients were clinically diagnosed with kernicterus after evaluation at the Kernicterus Clinic while eight were determined not to have kernicterus after clinical evaluation. When compared to the KSD-TK’s prediction, all 14 patients with KSD-TK “definite” kernicterus and 14 of the 15 patients with KSD-TK “probable” kernicterus were clinically diagnosed with kernicterus (Table 2). The patient with KSD-TK “probable” kernicterus who was incorrectly classified had a score of 6, the lowest possible score to be included as “probable” rather than “possible” kernicterus. One of the two patients with KSD-TK “possible” kernicterus (score of 5) was clinically diagnosed with kernicterus while the other patient (score of 4) was not. None of the six patients with KSD-TK “not kernicterus” were clinically diagnosed with kernicterus. When KSD-TK “definite” and “probable” were combined and “possible” and “not kernicterus” were combined, the KSD-TK had a sensitivity of 96.6% (28/29) in correctly predicting a clinical diagnosis of kernicterus and a specificity of 87.5% (7/8) in detecting the absence of kernicterus in patients who truly do not have the disorder.
When considering the value of using each individual risk factor from the KSD-TK for diagnosis, logistic regression suggested that all of the risk factors had high classification accuracy and sensitivity, and area under the ROC curve was >0.5 (Table 3). While the sensitivity was high for every risk factor, only three risk factors had a specificity ≥0.75: presence of ANSD, dystonia or abnormal movements on the last follow-up exam, and abnormal globus pallidus ± subthalamic nucleus on MRI results. These factors also had an area under the ROC curve of 0.86–0.97, indicating that they are all good measures to distinguish patients with and without kernicterus. Overall, ANSD had the best model fit statistics; however, the logarithm of the odds was unstable as indicated by the large standard error. This occurred because none of the patients designated as “not kernicterus” on the KSD-TK had a diagnosis of ANSD and therefore all of their values were 0 for this variable.
Discussion
Kernicterus is a classic cause of dyskinetic CP and presents as (1) excessive abnormal movements and tone (dystonia) ± athetosis (slow, writhing movements, a.k.a. choreoathetosis) due to lesions in the globus pallidus (GP); (2) auditory neuropathy spectrum disorder (ANSD) due to lesions in auditory brainstem nuclei ± nerve, and (3) oculomotor impairments.9 We have recently described motor-predominant and auditory-predominant subtypes as part of a continuum of kernicterus spectrum disorders.1
The high sensitivity and specificity of the KSD-TK indicate that it is a promising screening tool to determine the likelihood of KSD diagnosis and could allow for earlier evaluation of a patient with suspected KSD by a qualified provider as well as earlier treatment. Furthermore, the high sensitivity of each individual risk factor included on the KSD-TK supports the use of these measures in determining a KSD diagnostic prediction. The specificity ≥0.75 of ANSD, specific abnormal MRI brain results, and dystonia or abnormal movements on last follow-up exam indicate that these factors are the most predictive of KSD. This toolkit can be used on any child who is outside of the neonatal period, provided they have the necessary testing and clinical results.
When reviewing the patients included in the study, we found that only 2 of the 37 patients were incorrectly classified by the KSD-TK. Only one of eight patients who were not clinically diagnosed with kernicterus was incorrectly classified “probable” kernicterus by the KSD-TK. This patient had an elevated peak bilirubin of 32 mg/dL (2 points), an abnormal neonatal exam due to opisthotonus (arching of the back and neck) and high-pitched cry (2 points), abnormal last follow-up exam due to mild dystonia and hypertonicity (1 point), and probable abnormal MRI results due to subtle hyperintensity in the globus pallidus bilaterally (1 point) for total KSD-TK score of 6. This score is at the lowest end of the range for “probable” kernicterus and just one point above “possible” kernicterus. This patient’s severe encephalopathy was ultimately not clinically attributed to kernicterus because many of the patient’s abnormal features were not characteristic of kernicterus including spasticity, sensorineural hearing loss that did not fit the ANSD pattern, additional abnormalities on MRI including atrophy and hydrocephalus ex-vacuo, multiple episodes of pneumonia, a lack of the attentiveness, interaction and awareness that most patients with even severe kernicterus maintain and, finally, seizures continuing past a few months of age, when most seizures secondary to kernicterus resolve. Similarly, only 1 of 29 patients who were clinically diagnosed with kernicterus was classified incorrectly as “possible” kernicterus. This patient had an elevated peak bilirubin of 33 mg/dL (2 points), lethargy (1 point), mild dystonia and athetosis (1 point), mild ANSD with abnormal ABRs (1 point), and mild posterior lateral ventriculomegaly without lesions in the globus pallidus on MRI (0 points) for a total score of 5. This score is on the highest end of the range for “possible” kernicterus and only 1 point away from “probable” kernicterus. This patient was ultimately determined to have mild classical kernicterus based on clinical evaluation due to neonatal history, mild dystonia and athetosis, and mild ANSD. These two examples emphasize the importance of evaluation by a qualified physician after KSD-TK diagnostic prediction for both final diagnosis and potential treatment, while still acknowledging the value of the KSD-TK in providing families with an early diagnostic prediction to guide follow-up.
Limitations of this study include an unbalanced study sample in that, out of 37 patients, 29 were clinically diagnosed with kernicterus, while only 8 patients did not have a kernicterus diagnosis. This selection bias occurred because our patient sample consisted of children who were referred to and evaluated at our Kernicterus Clinic due to high suspicion for kernicterus and therefore were more likely to have kernicterus. Consequently, the reported prediction parameters (sensitivity, specificity, and area under ROC curve) may be an overestimate.
However, the increased incidence of kernicterus worldwide serves to emphasize the importance of a validated kernicterus screening tool. Worldwide, 24 million infants are at risk for neonatal hyperbilirubinemia-related adverse outcomes.2 The incidence of severe neonatal jaundice (bilirubin ≥25 mg/dL) is estimated to be 244/10,000 live births in low- and middle-income countries versus 4/10,000 in high-income countries.4 Without surveillance and measures to prevent severe hyperbilirubinemia 8/1000 term and 42/1000 preterm infants worldwide develop kernicterus13 and account for about 10% of all cases of cerebral palsy (CP). Recent estimates are that worldwide 56/100,000 live births (~1:1800) develop kernicterus with 73/100,000 (~1:1400) in eastern Europe, Asia, Latin America and Africa.2 Even in the United States, where kernicterus is considered to be essentially eliminated, an estimated incidence of 5/100,000 would result in 200 new cases of kernicterus per year in the USA.2,14,15 This underscores that while kernicterus may be forgotten, it is not gone, and the KSD-TK can bridge gaps in the recognition and diagnosis of affected children.
Whereas the KSD-TK may be a useful tool to suggest the diagnosis of a KSD in under-resourced areas, we recognize that our current KSD-TK relies in part on information that may not be available in under-resourced countries where the incidence of KSDs is high. Namely, MRI scans and ABRs to diagnose ANSD may not be available, and blood and/or transcutaneous bilirubin levels may not be available in many cases. Modifications of the original bilirubin-induced neurologic dysfunction (BIND) scoring algorithm designed to diagnose acute bilirubin encephalopathy (ABE) in resource-limited settings found that the modified BIND-M score was reliable and useful for predicting the development and severity of ABE in neonates.12 Similarly, we hope that our KSD-TK or a modification thereof can be validated to screen for KSDs in under-resourced settings.
Future studies to validate these results should include completion of the KSD-TK by parents of patients, outside nurses, or outside clinicians who are less familiar with KSDs in order to determine if the KSD-TK accurately predicts KSDs in these settings as well. While the project member completing the KSD-TK for these patients did not determine the patients’ clinical diagnosis, she did have the knowledge and resources about KSDs, and this could be a contributing bias that should be eliminated in future studies. Furthermore, future multi-center studies in a larger patient population, potentially spanning multiple countries where kernicterus is more common, could be designed to recruit equal numbers of patients in each group and allow for a more balanced sample. A modified KSD-TK without MRI, ABR, and bilirubin levels and with a greater emphasis on clinical findings could be created and validated in limited resource areas. These data could be combined with our previously published KSD-TK2 detailing kernicterus subtype and severity1 to create a validated Kernicterus Registry and track incidence, prevalence, natural history, and responses to various treatments16 regarding the kernicterus spectrum disorders.
Conclusion
The KSD-TK is an effective screening tool for parents and clinicians. Based on the data, it is an accurate diagnostic predictor with a sensitivity of 97% and a specificity of 87%. Furthermore, each individual risk factor included in the toolkit showed a high classification accuracy, supporting the use of each risk factor in the diagnostic toolkit. Utilizing the KSD-TK as a standardized screening tool could lead to more prompt diagnoses, fewer missed diagnoses, and earlier treatment, while also enhancing our understanding of the incidence, prevalence and natural history of KSDs. Future prospective studies can be done with larger sample sizes, different populations, and using KSD-TK predictions completed by external physicians and caregivers, which will further corroborate this data.
References
Le Pichon, J. B., Riordan, S. M., Watchko, J. & Shapiro, S. M. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the Kernicterus Spectrum Disorders (Ksds). Curr. Pediatr. Rev. 13, 199–209 (2017).
Bhutani, V. K. et al. Neonatal hyperbilirubinemia and rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr. Res. 74, 86–100 (2013).
Olusanya, B. O., Ogunlesi, T. A. & Slusher, T. M. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch. Dis. Child. 99, 1117–1121 (2014).
Slusher, T. M. et al. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr. Open 1, e000105 (2017).
Sgro, M., Campbell, D. M., Kandasamy, S. & Shah, V. Incidence of chronic bilirubin encephalopathy in Canada, 2007−2008. Pediatrics 130, e886–890 (2012).
Martin, J. A. et al. Births: final data for 2007. Natl Vital-. Stat. Rep. 58, 1–85 (2010).
Das, S. & van Landeghem, F. K. H. Clinicopathological spectrum of bilirubin encephalopathy/kernicterus. Diagnostics 9, 1–12 (2019).
Hansen, T. W. Kernicterus: an international perspective. Semin. Neonatol. 7, 103–109 (2002).
Shapiro, S. M. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin. Fetal Neonatal Med. 15, 157–163 (2010).
Okumura, A. et al. Kernicterus in preterm infants. Pediatrics 123, e1052–1058 (2009).
Zhang, F., Chen, L., Shang, S. & Jiang, K. A clinical prediction rule for acute bilirubin encephalopathy in neonates with extreme hyperbilirubinemia: a retrospective cohort study. Medicine 99, e19364 (2020).
Radmacher, P. G. et al. A modified bilirubin-induced neurologic dysfunction (Bind-M) algorithm is useful in evaluating severity of jaundice in a resource-limited setting. BMC Pediatr. 15, 28 (2015).
Crosse, V. M., Meyer, T. C. & Gerrard, J. W. Kernicterus and prematurity. Arch. Dis. Child. 30, 501–508 (1955).
Sgro, M., Campbell, D. & Shah, V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. Cmaj 175, 587–590 (2006).
Ebbesen, F. Recurrence of kernicterus in term and near-term infants in Denmark. Acta Paediatr. 89, 1213–1217 (2000).
Shapiro, S. M. & Riordan, S. M. Review of bilirubin neurotoxicity Ii: preventing and treating acute bilirubin encephalopathy and kernicterus spectrum disorders. Pediatr. Res. 87, 332–337 (2020).
Author information
Authors and Affiliations
Contributions
V.R.D. contributed to the conception and design of the study, interpretation of data, revising the article, and approved the final version. S.M.S. contributed to the conception and design of the study, conception and writing the original Kernicterus Spectrum Disorders Diagnostic Toolkit, interpretation of data, revising the article, and approved the final version. H.-W.Y. contributed to data analysis and interpretation and approved the final version. R.G.-M. contributed to the conception and design of the study, interpretation of data, revising the article, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dasari, V.R., Shapiro, S.M., Yeh, HW. et al. Kernicterus Spectrum Disorders Diagnostic Toolkit: validation using retrospective chart review. Pediatr Res 91, 862–866 (2022). https://doi.org/10.1038/s41390-021-01755-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01755-5
This article is cited by
-
Predictive and diagnostic measures for kernicterus spectrum disorder: a prospective cohort study
Pediatric Research (2023)
-
Bilirubin Encephalopathy
Current Neurology and Neuroscience Reports (2022)