Abstract
Background
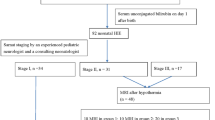
Kernicterus spectrum disorder (KSD) resulting from neonatal hyperbilirubinemia remains a common cause of cerebral palsy worldwide. This 12-month prospective cohort study followed neonates with hyperbilirubinemia to determine which clinical measures best predict KSD.
Methods
The study enrolled neonates ≥35 weeks gestation with total serum bilirubin (TSB) ≥ 20 mg/dl admitted to Aminu Kano Hospital, Nigeria. Clinical measures included brain MRI, TSB, modified bilirubin-induced neurologic dysfunction (BIND-M), Barry-Albright Dystonia scale (BAD), auditory brainstem response (ABR), and the modified KSD toolkit. MRI signal alteration of the globus pallidus was scored using the Hyperbilirubinemia Imaging Rating Tool (HIRT).
Results
Of 25 neonates enrolled, 13/25 completed 12-month follow-up and six developed KSD. Neonatal BIND-M ≥ 3 was 100% sensitive and 83% specific for KSD. Neonatal ABR was 83% specific and sensitive for KSD. Neonatal HIRT score of 2 was 67% sensitive and 75% specific for KSD; this increased to 100% specificity and sensitivity at 12 months. BAD ≥ 2 was 100% specific for KSD at 3–12 months, with 50–100% sensitivity.
Conclusions
Neonatal MRIs do not reliably predict KSD. BIND-M is an excellent screening tool for KSD, while the BAD or HIRT score at 3 or 12 months can confirm KSD, allowing for early diagnosis and intervention.
Impact
-
The first prospective study of children with acute bilirubin encephalopathy evaluating brain MRI findings over the first year of life.
-
Neonatal MRI is not a reliable predictor of kernicterus spectrum disorders (KSD).
-
Brain MRI at 3 or 12 months can confirm KSD.
-
The modified BIND scale obtained at admission for neonatal hyperbilirubinemia is a valuable screening tool to assess risk for developing KSD.
-
The Barry Albright Dystonia scale and brain MRI can be used to establish a diagnosis of KSD in at-risk infants as early as 3 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Le Pichon, J. B., Riordan, S. M., Watchko, J. & Shapiro, S. M. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDS). Curr. Pediatr. Rev. 13, 199–209 (2017).
Mcgillivray, A. & Evans, N. Severe neonatal jaundice: is it a rare event in Australia? J. Paediatr. Child Health 48, 801–807 (2012).
Sgro, M., Campbell, D. M., Kandasamy, S. & Shah, V. Incidence of chronic bilirubin encephalopathy in Canada, 2007–2008. Pediatrics 130, e886–e890 (2012).
Donneborg, M. L., Hansen, B. M., Vandborg, P. K., Rodrigo-Domingo, M. & Ebbesen, F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in denmark during the years 2000-2015. J. Perinatol. 40, 194–202 (2020).
Alkén, J., Håkansson, S., Ekéus, C., Gustafson, P. & Norman, M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw. Open 2, e190858 (2019).
Olusanya, B. O., Kaplan, M. & Hansen, T. W. R. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc. Health 2, 610–620 (2018).
Iskander, I. & Gamaleldin, R. Acute bilirubin encephalopathy: some lessons learned. Semin Perinatol. 45, 151353 (2021).
Banerjee, T. K. et al. Neurological disorders in children and adolescents. Indian J. Pediatr. 76, 139–146 (2009).
Jibril, Y. N. et al. Determinants of hearing loss in children with cerebral palsy in Kano, Nigeria. Niger. J. Clin. Pr. 24, 802–807 (2021).
Okenwa, W. & Edeh, A. A review of clinical presentation and physiotherapy management of cerebral palsy patients in Esut teaching hospital, Enugu, Nigeria. Afr. Health Sci. 19, 3085–3090 (2019).
Slusher, T. M. et al. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr. Open 1, e000105 (2017).
Bhutani, V. K. et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr. Res 74, 86–100 (2013).
Novak, I. et al. Early, accurate diagnosis and early intervention in cerebral palsy. JAMA Pediatr. 171, 897 (2017).
Radmacher, P. G. et al. A modified bilirubin-induced neurologic dysfunction (Bind-M) algorithm is useful in evaluating severity of jaundice in a resource-limited setting. BMC Pediatr. 15, 28 (2015).
Slusher, T. M., Owa, J. A., Painter, M. J. & Shapiro, S. M. The kernicteric facies: facial features of acute bilirubin encephalopathy. Pediatr. Neurol. 44, 153–154 (2011).
Johnson, L., Bhutani, V. K., Karp, K., Sivieri, E. M. & Shapiro, S. M. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J. Perinatol. 29, S25–S45 (2009).
Lai, N. M., Gerard, J. P., Ngim, C. F., Kamar, A. A. & Chen, K.-H. The association between serum bilirubin and kernicterus spectrum disorder: a systematic review and meta-analysis. Neonatology 118, 654–664 (2021).
Shapiro, S. M. & Popelka, G. R. Auditory impairment in infants at risk for bilirubin-induced neurologic dysfunction. Semin Perinatol. 35, 162–170 (2011).
Dasari, V. R., Shapiro, S. M., Yeh, H.-W. & Gelineau-Morel, R. Kernicterus spectrum disorders diagnostic toolkit: validation using retrospective chart review. Pediatr. Res. 91, 862–866 (2022).
Barry, M. J., VanSwearingen, J. M. & Albright, A. L. Reliability and responsiveness of the Barry-Albright dystonia scale. Dev. Med Child Neurol. 41, 404–411 (1999).
Yilmaz, Y. et al. Magnetic resonance imaging findings in patients with severe neonatal indirect hyperbilirubinemia. J. Child Neurol. 16, 452–455 (2001).
Kitai, Y. et al. Diagnosis of bilirubin encephalopathy in preterm infants with dyskinetic cerebral palsy. Neonatology 117, 73–79 (2020).
Ribeiro, B. N. D. F., Lima, G. D. A., Ventura, N., Gasparetto, E. L. & Marchiori, E. Chronic kernicterus: magnetic resonance imaging findings. Radiol. Brasileira 49, 407–408 (2016).
Martich-Kriss, V., Kollias, S. S. & Ball, W. S. Mr findings in kernicterus. AJNR Am. J. Neuroradiol. 16, 819–821 (1995).
Wisnowski, J. L., Panigrahy, A., Painter, M. J. & Watchko, J. F. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin. Perinatol. 38, 422–428 (2014).
Lequin, M., Groenendaal, F., Dudink, J. & Govaert, P. Susceptibility weighted imaging can be a sensitive sequence to detect brain damage in neonates with kernicterus: a case report. BMC Neurol. 23, 104 (2023).
Caksen, H. Brain magnetic resonance imaging and magnetic resonance spectroscopy findings of children with kernicterus. Pol. J. Radiol. 80, 72–80 (2015).
Wang, X. et al. Studying neonatal bilirubin encephalopathy with conventional Mri, Mrs, and Dwi. Neuroradiology 50, 885–893 (2008).
Wu, W., Zhang, P., Wang, X., Chineah, A. & Lou, M. Usefulness of<Sup>1</Sup>H-Mrs in differentiating bilirubin encephalopathy from severe hyperbilirubinemia in neonates. J. Magn. Reson. Imaging 38, 634–640 (2013).
Shapiro, S. M. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (Bind). J. Perinatol. 25, 54–59 (2005).
Shrout, P. E. & Fleiss, J. L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420–428 (1979).
Hall, P. The Bootstrap and Edgeworth Expansion (Springer, New York, 1992).
R Core Team. in R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2022).
in RStudio: Integrated Development Environment for R (RStudio, PBC, 2022).
Gamer, M., Lemon, J., Fellows, I. & Singh, P. (R package version 0.84.1, 2019).
Canty, A. & Ripley, B. (R package version 1.3–28, 2021).
Thulin, M. (R package version 0.4, 2021).
Torgo, L. Data Mining with R, Learning with Case Studies 2nd edn, (Chapman and Hall/CRC Press, 2017).
Cicchetti, D. V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 6, 284–290 (1994).
El Houchi, S. Z. et al. Prediction of 3- to 5-month outcomes from signs of acute bilirubin toxicity in newborn infants. J. Pediatr. 183, 51–55.e1 (2017).
Kang, W. et al. Early prediction of adverse outcomes in infants with acute bilirubin encephalopathy. Ann. Clin. Transl. Neurol. 7, 1141–1147 (2020).
Funding
This work was funded by a Children’s Mercy Hospital internal grant to J.B.L.P. R.G.-M. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number T32HD069038). The funding sources had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
R.G.-M. contributed to conceptualization, investigation, methodology, data curation, visualization, and writing the original draft. Fatima Usman contributed to conceptualization, investigation, data curation, and project administration. S.S., Y.J., and M.A. contributed to investigation and data curation. H.-W.Y. contributed to formal analysis and visualization. Mohammad Suwaid contributed to methodology, data curation, investigation, and project administration. K.S. contributed to data interpretation and visualization. S.S. contributed to conceptualization, investigation, and methodology. T.Z. and H.H. contributed to investigation. T.S., J.-B.L.P., and Z.F. contributed to conceptualization, methodology, and supervision. All authors contributed to writing- review and editing and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.G.-M. is supported by the NICHD (grant number T32HD069038). She has received a travel grant from the American Society of Clinical Pharmacology and Therapeutics and has a provisional patent for a novel drug for the treatment of movement disorders. F.U. and S.S. declared internal funding from Children’s Mercy Hospital to support patient MRI costs for the study. T.S. declared internal funding for travel and hotel only from Children’s Mercy Hospital related to this study. J.-B.L.P. declared internal funding from Children’s Mercy Hospital to support the costs of this study. He also serves on the editorial board of Pediatric Neurology and Annals of Child Neurology Society and is a clinical advisory round table member for the Firefly Fund for Neimann Pick type C. J.-B.L.P. also provides medical expertize on an occasional basis to various law firms. All other authors declare no conflicts of interest.
Informed consent
Participant families provided informed consent for this study through the Aminu Kano Teaching Hospital, Nigeria.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gelineau-Morel, R., Usman, F., Shehu, S. et al. Predictive and diagnostic measures for kernicterus spectrum disorder: a prospective cohort study. Pediatr Res 95, 285–292 (2024). https://doi.org/10.1038/s41390-023-02810-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02810-z