Abstract
Background
Therapeutic hypothermia is a standard of care for neonatal encephalopathy; however, approximately one in two newborn infants fails to respond to this treatment. Recent studies have suggested potential relationships between body temperature, heart rate and the outcome of cooled infants.
Methods
The clinical data of 756 infants registered to the Baby Cooling Registry of Japan between January 2012 and December 2016 were analysed to assess the relationship between body temperature, heart rate and adverse outcomes (death or severe impairment at 18 months corrected age).
Results
A lower body temperature at admission was associated with adverse outcomes in the univariate analysis (P < 0.001), the significance of which was lost when adjusted for the severity of encephalopathy and other covariates. A higher body temperature during cooling and higher heart rate before and during cooling were associated with adverse outcomes in both univariate (all P < 0.001) and multivariate (P = 0.012, P < 0.001 and P < 0.001, respectively) analyses.
Conclusions
Severe hypoxia–ischaemia might be a common causative of faster heart rates before and during cooling and low body temperature before cooling, whereas causal relationships between slightly higher temperatures during cooling and adverse outcomes need to be elucidated in future studies.
Impact
-
In a large cohort of encephalopathic newborn infants, dual roles of body temperature to the outcome were shown; adverse outcomes were associated with a lower body temperature at admission and higher body temperature during cooling.
-
A higher heart rate before and during cooling were associated with adverse outcomes.
-
Severe hypoxia–ischaemia might be a common causative of faster heart rates before and during cooling and low body temperature before cooling.
-
The exact mechanism underlying the relationship between slightly higher body temperature during cooling and adverse outcomes remains unknown, which needs to be elucidated in future studies.
Similar content being viewed by others
Introduction
Therapeutic hypothermia is the current standard of care for near-term and term newborn infants with moderate-to-severe hypoxic–ischaemic encephalopathy.1 Hypothermia reduces death and neurological impairments of encephalopathic infants by 18 months of age.2 However, 35–55% of survivors still develop neurological impairments.3,4 Clinical variables associated with the efficacy of cooling have been rigorously investigated because it may help improve the benefits of cooling via the identification of novel therapeutic targets and patients who are more likely to respond to treatment. Thus far, the potential adverse impact of clinical conditions, such as hypocarbia,5 small body size,6 smaller gestational age,7 abnormal glucose levels8 and pyrexia,9 on the outcomes of encephalopathic infants have been reported. However, the mechanisms explaining the relationships between these variables and outcomes remain mostly unknown. In addition, several established independent variables of the outcome in preclinical models, such as a delay in cooling initiation and subtle differences in the cooling level,10,11,12,13 have not been fully confirmed in clinical settings.
Using a large-scale data set from the Baby Cooling Registry of Japan, a national registry of cooled infants, we previously identified novel clinical factors associated with the survival to discharge of cooled infants by day 28,14 such as higher body temperatures at admission, lower body temperatures (mostly within the recommended cooling temperature range) during cooling and slower heart rates before, during and after cooling. Tachycardia during cooling is increasingly recognised as a potential independent variable associated with adverse outcomes in medium-sized cohorts of adults after cardiac arrest15 and asphyxiated newborn infants.16,17 In contrast, conflicting observations have been reported regarding the relationship between body temperature and outcomes. Based on preclinical studies, which consistently showed the exponential loss of cerebral protection with delays in cooling,10,12,13 clinical researchers addressed the early initiation of cooling in newborn infants. Laptook et al. assessed the efficacy of delayed cooling in newborn infants and found that the benefit of cooling commenced between 6 and 12 h of birth is likely to be less robust compared with that of early cooling within 6 h of birth.18 Thoresen et al. observed better developmental quotients at 18–20 months in 35 infants cooled ≤3 h after birth as opposed to 30 infants cooled > 3 h after birth.19 However, secondary analyses from large-scale studies did not find any outcomes dependent on the timing of cooling initiation.20,21 Wood et al. demonstrated in a rat pup model of hypoxia–ischaemia that spontaneous low body temperature < 32.2 °C before the commencement of cooling is associated with more severe brain injury, whereas active cooling to 32 °C ameliorated brain injury compared with normothermic temperature management.22 These findings may suggest that, while cooling with minimum delay is likely to provide the maximum benefit, early completion of cooling induction might imply a mixed consequence of reduced thermogenesis due to severe hypoxia–ischaemia and early, efficient cooling. Regarding the target body temperature during 72 h cooling, the short-term mortality of infants, who were cooled to 32 °C, was 14%, as opposed to 7% for those who were cooled to 33–34 °C (current recommendation), with no improvement in the long-term outcomes at 2 years.23,24 Further understanding of the relationship between body temperature and heart rate before and during cooling and long-term outcomes of cooled infants may help improve the selection of infants, prediction of outcomes and efficacy of cooling.
The primary aim of this study was to investigate the relationship between body temperature before and during cooling and the outcomes assessed at 18 months of age in a large cohort of cooled infants. The secondary aim was to investigate the relationship between heart rate before and during cooling and the same outcomes.
Materials and methods
Ethics approval and consent
This study was conducted in compliance with the Declaration of Helsinki. The protocols of the registry were approved by the Ethics Committees of Kurume University School of Medicine and Saitama Medical University, Japan. The ethics committees approved that parental consent was not needed for the registry, since no patient identifier was collected.
Population and data collection
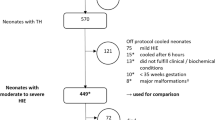
For this observational study, we reviewed the clinical data of all 776 cooled infants compiled between January 1, 2012 and December 31, 2016 (Table 1 and Fig. 1). We excluded 20 infants with major congenital abnormalities, chromosomal aberrations and/or brain dysgenesis. In total, 756 infants were included in the study.
The following clinical information was obtained from the registry database: heart rate, body temperature and the mean blood pressure at admission (heart rate and blood pressure were not collected at admission), 0 h, 24 h and mean values between 3 and 72 h after the commencement of cooling (original data collected every 3 h between 0 and 12 h of cooling and every 12 h between 12 and 72 h of cooling), gestational age, birth weight, birth location, 10-min Apgar score, pH and base deficit of cord or first blood gas sample obtained < 1 h after birth, Sarnat encephalopathy stage at admission,25 Thompson encephalopathy scores26 at admission and 24 h after initiating cooling, cooling practice, and time to target body temperature after birth.
Assessment of outcomes
The outcomes of cooled infants were assessed at 18 (16–20) months post-conceptional age by a consultant neonatologist, paediatrician or child neurologist. The presence of hearing loss or blindness, as well as the need for tube feeding and/or respiratory support were noted. Neuromotor function was classified using the Gross Motor Function Classification System (GMFCS),27 which gives a score of 0–5, with higher scores indicating greater impairments. An adverse outcome was defined as death or survival with severe disability, such as tube feeding and/or respiratory support, hearing loss, blindness and cerebral palsy (GMFCS score > 2).
Data analysis
Each clinical record was inspected for case duplication, apparent input errors or missing data > 5% of the individual data set without plausible explanations. To assess the influence of follow-up loss, clinical backgrounds were compared between infants with and without confirmed outcomes at 18 months of age. To reduce attrition biases due to missing data, multiple imputation of independent variables was performed (n = 5 imputations) based on the correlation between variables with missing values and other subject characteristics (SPSS ver. 26; IBM, Armonk, NY). To identify the crude effect of clinical backgrounds and therapeutic options on the outcome, variables were compared between infants with favourable and adverse outcomes using Student’s t test, Mann–Whitney U test or chi-squared test, where appropriate. P values < 0.002 were considered significant after correcting for multiple comparisons over 21 variables. Body temperatures during whole-body cooling and selective-head cooling were also compared using Student’s t test.
The dependence of the outcome on body temperature (and then, on heart rate) before and during cooling was assessed using logistic regression analysis. For body temperature before cooling, temperature values at admission were used to best represent the level before starting active temperature control. Because heart rates at admission were not collected, heart rates at initiating cooling were used to represent the values before cooling. For body temperature and heart rate during cooling, mean values between 3 and 72 h of cooling were used. Findings are also presented with/without correction for covariates, which were chosen based on priori hypotheses for each independent variable. Body temperature and heart rate before cooling were adjusted for gestational age, birth location and encephalopathy stage at admission (and additionally by body temperature and mean blood pressure for the heart rate). Body temperature and heart rate during cooling were adjusted for gestational age, encephalopathy stage at admission and cooling practice (and additionally by body temperature and mean blood pressure between 3 and 72 h of cooling for the heart rate).
Results
Of the 756 participants, outcomes at 18 months of age were confirmed in 604 infants (final study cohort). These infants had less outborn infants (68.5%) compared with those who were lost to follow-up (80.8% of 152 infants; P = 0.003); however, other background variables of gestational age, birth weight, 10-min Apgar scores, first blood gas pH and base deficit, Thompson encephalopathy score and Sarnat encephalopathy stage were similar between these infants (Online Supplemental Table 1).
Of the infants within the final study cohort, 591 (97.8%) were confirmed to have been cooled using electronic, temperature-controlled cooling devices (Online Supplemental Table 2). The body temperature during selective-head cooling (mean, 34.0 °C; standard deviation, 0.5 °C; n = 178) was higher than that during whole-body cooling (mean, 33.7 °C; standard deviation, 0.4 °C; n = 426) (P < 0.001, Online Supplemental Table 2). At 18 months corrected age, 45 infants (7.5%) had died and 133 infants (23.8%) had developed severe impairments (Table 1 and Fig. 1). The severe impairments of survivors were: dependence on tube feeding (n = 86, 15.4%), dependent on respiratory support (n = 48, 8.6%), hearing loss (n = 36, 6.4%), blindness (n = 18, 3.2%), and cerebral palsy (n = 116, 20.5%). Infants with adverse outcomes had lower 10-min Apgar scores and first blood gas pH, greater first blood gas base deficit, higher Thompson scores (at admission and 24 h after cooling initiation), greater Sarnat encephalopathy stage (at admission), faster heart rates (at cooling initiation, 24 h and mean values between 3 and 72 h after cooling initiation), lower body temperatures at admission and cooling initiation and higher body temperatures at 24 h and mean values between 3 and 72 h after cooling initiation (all P < 0.001; Table 2 and Fig. 2a, b). There was a trend that the time to target temperature after birth was relatively shorter for those with adverse outcomes than their peers with favourable outcomes (P = 0.013).
Adverse outcomes of newborn infants were associated with lower body temperatures at admission and higher body temperatures during cooling (a; both P < 0.001, univariate logistic regression analysis). Higher heart rates before and during cooling were consistently related with adverse outcomes of the infants (b; both P < 0.001, univariate logistic regression analysis). Values are shown as mean (95% confidence interval). Data at admission were not collected for the heart rate.
Relationships between body temperature and adverse outcomes
In the univariate analysis, lower body temperatures at admission were associated with adverse outcomes (odds ratio [OR], 0.67; 95% confidence interval [CI], 0.57–0.78; P < 0.001), the significance of which was lost after adjusting for gestational age, birth location and Sarnat encephalopathy stage at admission (OR, 0.84; 95% CI, 0.70–1.02; P = 0.076) (Table 3). In contrast, higher body temperatures during cooling were associated with adverse outcomes in both the univariate analysis (OR, 2.14; 95% CI, 1.43–3.20; P < 0.001) and multivariate analysis, which was adjusted for gestational age, Sarnat encephalopathy stage at admission and cooling practice (OR, 1.97; 95% CI, 1.17–3.34; P = 0.012) (Table 3).
Relationships between heart rate and adverse outcomes
Faster heart rates at cooling initiation were associated with adverse outcomes in both the univariate analysis (OR, 1.19; 95% CI, 1.08–1.31, per 10 beats/min [bpm]; P < 0.001) and multivariate analysis, which was adjusted for gestational age, birth location, Sarnat encephalopathy stage at admission, body temperature and the mean blood pressure at cooling initiation (OR, 1.29; 95% CI, 1.12–1.48, per 10 bpm; P < 0.001) (Table 3). Faster heart rates during cooling were also associated with adverse outcomes in both the univariate analysis (OR, 2.31; 95% CI, 1.94–2.75, per 10 bpm; P < 0.001) and multivariate analysis, which was adjusted for gestational age, Sarnat encephalopathy stage at admission, cooling practice, body temperature and the mean blood pressure during cooling (OR, 1.98; 95% CI, 1.61–2.44, per 10 bpm; P < 0.001) (Table 3).
Discussion
In a large cohort of encephalopathic infants, body temperatures before and during therapeutic hypothermia showed entirely different relationships with outcomes at 18 months corrected age, where adverse outcomes were associated with lower body temperatures at admission and higher body temperatures during cooling. Building on previous studies in adults and newborn infants, which suggested a relationship between faster heart rates during cooling and adverse outcomes following hypoxic–ischaemic brain injury,14,15,16,17 our study also observed a consistent relationship between faster heart rates before and during cooling and adverse outcomes.
Body temperature at admission and outcome
Preclinical studies consistently supported the benefit of early cooling to maximise its neuroprotective effect.10,12,13 To minimise the delay in cooling, pre-hospital cooling has been proposed for both newborn infants and adults.28,29 Passive cooling during transportation facilitates early induction of therapeutic hypothermia in newborn infants30,31; however, thus far, no clinical data support the long-term benefit of pre-hospital cooling. Retrospective analyses of large-scale clinical trials have also failed to demonstrate a relationship between the timing of cooling initiation and outcome of cooled infants.20,21 In the univariate analysis of our study, a lower body temperature at admission was rather associated with an adverse outcome. In addition, a shorter elapsed time from birth to reach the target cooling temperature showed a modest relationship with adverse outcomes (Table 2). These findings may in part explain the reason why early induction of cooling has not been demonstrated to improve outcomes in clinical settings. Given that the relationship between body temperatures at admission and outcomes in our study was lost when adjusted for covariates including the severity of encephalopathy, low body temperatures at admission may represent the reduced heat production following severe hypoxia–ischaemia. After hypoxic–ischaemic events, spontaneous downregulation of thermogenesis (behavioural hypothermia) has been recognised in a range of species,22,32 temperature reduction of which depends on the severity of hypoxic–ischaemic encephalopathy in newborn infants.33 Although behavioural hypothermia has been considered a response to provide cerebral protection and ensure survival, our current study found that a lower body temperature at admission was associated with an adverse outcome, suggesting that either the benefit of the protective response was insufficient or temperature reduction was merely a consequence of severe injury. Our previous analysis from the same registry consistently showed that low body temperature at admission was dependent on the severity of hypoxia–ischaemia (i.e. greater first blood base deficit and Thompson encephalopathy scores), variables related with thermogenesis and heat loss (i.e. smaller birth weight and being outborn) and, in outborn infants only, the target body temperature during transportation,14 suggesting that the body temperature at admission is affected by both intrinsic and extrinsic conditions. With regards to early cooling during transportation, complications have been reported including excessive cooling and hypocarbia.34,35 A study in Ghana reported that the depth of induced passive cooling out of an intensive care setting correlated with adverse short-term outcomes of infants.33 Although we speculate that severe hypoxia–ischaemia is a common upstream event of spontaneous temperature reduction and subsequent adverse outcomes observed in our current study, further accumulation of clinical evidence is required to preclude the possibility that hypothermia induced before the commencement of intensive care is deleterious.
Body temperature during cooling and outcome
Pre-clinical studies suggested that the temperature range that provides optimal brain protection may vary according to the model and species.11,36 Little is known regarding the optimal cooling level for asphyxiated newborn infants. A recent large-scale trial suggested that cooling to 32.0 °C for 72 h led to a short-term mortality rate of 17%, as opposed to 7% for those who were cooled to the currently recommended level of 33.5 °C for 72 h, with no improvement in the outcomes at 2 years of age.23,24 Although cooling < 33.0 °C is unlikely to provide additional benefit, our study demonstrated that higher body temperatures during cooling were associated with adverse outcomes. Considering that a slightly higher body temperature of 34–35 °C has been recommended for selective-head cooling than that of 33–34 °C for whole-body cooling,37,38,39,40 selective-head cooling might be responsible for slightly higher body temperatures during cooling and subsequent adverse outcomes. However, in our current cohort, although the mean body temperature during selective-head cooling was higher than that during whole-body cooling, the relationship between the cooling practice and outcomes was not observed. In addition to the target cooling temperature, pyrexia and poor temperature control during cooling may also explain the relationship between slightly higher body temperatures and adverse outcomes,9 which might occur with the use of non-servo-controlled cooling devices, such as ice/water bags and ambient temperature control. However, in our current study, at least 97.8% of infants were confirmed to have been cooled using electronic, temperature-controlled cooling devices (Online Supplemental Table 2). Future studies need to address the exact relationship between the insult severity, body temperature during cooling and outcomes.
Heart rate and outcome
Bradycardia under hypothermia is a physiological response to reduced metabolic demand.15 In newborn infants, body temperature reduction by 1 °C induces heart rate reduction by approximately 10 bpm.41 However, Stær-Jensen et al.15 reported that faster heart rates during cooling in adults with out-of-hospital cardiac arrest were associated with adverse outcomes. Recently, two studies of encephalopathic infants suggested that faster heart rates during cooling were associated with adverse long-term outcomes.16,17 Our study extensively confirmed in a large study population that infants who subsequently developed adverse outcomes were faster heart rates before and during cooling. Faster heart rates by 10 bpm at the commencement of cooling and during cooling were associated with approximately 1.3–2.0-fold increases in OR of developing adverse outcomes. Tachycardia can be observed as a physiological response to pyrexia42 and hypovolaemia.43 Our previous analysis from the same registry suggested that higher body temperatures are associated with faster heart rates before and during cooling.14 However, in our current study, the relationship between heart rate and outcomes remained significant even when adjusted for body temperature and blood pressure, suggesting that faster heart rates may be, at least in part, a consequence of severe hypoxia–ischaemia. The relationship between faster heart rates and adverse outcomes might be explained as a compensatory reaction to myocardial dysfunction and reduced cardiac output following severe hypoxia–ischaemia.44 However, we were unable to incorporate the information regarding the cardiac function and use of inotropes and volume expanders. It is also possible that physiological regulation of cerebral perfusion coupled with its metabolic demand was not preserved following severe injuries. Studies are required to assess the dependence of outcomes on the cardiac and brainstem functions following perinatal hypoxia–ischaemia.
Limitations
Our study was based on a retrospective analysis of a prospectively recruited cohort. Although the Baby Cooling Registry is a national registry, in which approximately 70% of Japanese level 2–3 neonatal intensive care units participated, biases may exist regarding the enrolment of cooled infants at each participating centre. Also, like most other observational studies, it is difficult to translate the observed relationships between clinical variables and outcomes into causal relationships. Therefore, the potential roles of body temperature and heart rate before and during cooling on the outcomes need to be delineated in future prospective studies, which control for confounders such as cardiac function, use of vasopressors and volume expanders and temperature management during transportation and cooling. Second, we were unable to use cognitive outcomes as the primary endpoint of the study, because individualised neuro-developmental assessments were not performed on all infants. Third, stage III encephalopathy was identified in 23.8% of the entire cohort, who accounted for 61.6% of the infants with adverse outcomes, suggesting that our findings might predominantly reflect the nature of most severely affected infants. Fourth, although temperature monitoring sites are known to affect the body temperature reading,45 we were unable to incorporate this information within the analysis because the primary temperature monitoring site was not confirmed in 9.1% of the infants. Finally, the Japanese cultural background considerably differs from those in Western countries. As reported previously,46,47 withdrawal of life support is uncommon in Japan, potentially leading to relatively higher rates of survivors with neurological impairments. Careful consideration is required when generalising our findings to other parts of the world.
Conclusions
In asphyxiated newborn infants, lower body temperatures at admission and higher body temperatures during cooling were associated with adverse outcomes at 18 months of age. Faster heart rates before and during active cooling were consistently associated with adverse outcomes. These findings may help further our understanding of the injury cascade following hypoxia–ischaemia and improve the prediction of outcomes. Future studies need to address the roles of spontaneous and induced hypothermia before the commencement of intensive care on outcomes and identify the causal relationship between relatively higher body temperatures during cooling and outcomes.
References
Perlman, J. M. et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 132, S204–S241 (2015).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, CD003311 (2013).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Azzopardi, D. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 371, 140–149 (2014).
Pappas, A. et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 158, 752–758 e751 (2011).
Wyatt, J. S. et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 119, 912–921 (2007).
Rao, R. et al. Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34-35 weeks gestational age with hypoxic-ischemic encephalopathy. J. Pediatr. 183, 37–42 (2017).
Basu, S. K. et al. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy: a post hoc analysis of the CoolCap Study. Arch. Dis. Child. Fetal Neonatal Ed. 101, F149–F155 (2016).
Laptook, A. R. et al. Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann. Neurol. 73, 520–528 (2013).
Davidson, J. O. et al. How long is too long for cerebral cooling after ischemia in fetal sheep? J. Cereb. Blood Flow Metab. 35, 751–758 (2015).
Gunn, A. J. et al. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Investig. 99, 248–256 (1997).
Gunn, A. J. et al. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 102, 1098–1106 (1998).
Sabir, H., Scull-Brown, E., Liu, X. & Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 h in neonatal rats. Stroke 43, 3364–3370 (2012).
Tsuda, K. et al. Body temperature, heart rate, and short-term outcome of cooled infants. Ther. Hypothermia Temp. Manag. 9, 76–85 (2019).
Staer-Jensen, H. et al. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit. Care Med. 42, 2401–2408 (2014).
Elstad, M., Liu, X. & Thoresen, M. Heart rate response to therapeutic hypothermia in infants with hypoxic-ischaemic encephalopathy. Resuscitation 106, 53–57 (2016).
Montaldo, P. et al. Electrocardiographic and echocardiographic changes during therapeutic hypothermia in encephalopathic infants with long-term adverse outcome. Resuscitation 130, 99–104 (2018).
Laptook, A. R. et al. Effect of therapeutic hypothermia initiated after 6 h of age on death or disability among newborns with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 1550–1560 (2017).
Thoresen, M. et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 104, 228–233 (2013).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340, c363 (2010).
Tagin, M. A. et al. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 166, 558–566 (2012).
Wood, T. et al. Rectal temperature in the first five hours after hypoxia-ischaemia critically affects neuropathological outcomes in neonatal rats. Pediatr. Res. 83, 536–544 (2018).
Shankaran, S. et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA 312, 2629–2639 (2014).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 318, 57–67 (2017).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 86, 757–761 (1997).
Palisano, R. et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 39, 214–223 (1997).
Kendall, G. S. et al. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 95, F408–F412 (2010).
Kämäräinen, A. et al. Induction of therapeutic hypothermia during prehospital CPR using ice-cold intravenous fluid. Resuscitation 79, 205–211 (2008).
Lemyre, B. et al. Initiation of passive cooling at referring centre is most predictive of achieving early therapeutic hypothermia in asphyxiated newborns. Paediatr. Child Health 22, 264–268 (2017).
Roberts, C. T., Stewart, M. J. & Jacobs, S. E. Earlier initiation of therapeutic hypothermia by non-tertiary neonatal units in Victoria, Australia. Neonatology 110, 33–39 (2016).
Wood, S. C. & Gonzales, R. Hypothermia in hypoxic animals: mechanisms, mediators, and functional significance. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 113, 37–43 (1996).
Enweronu-Laryea, C. et al. Core temperature after birth in babies with neonatal encephalopathy in a sub-Saharan African hospital setting. J. Physiol. 597, 4013–4024 (2019).
Hallberg, B. et al. Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr. 98, 942–946 (2009).
Szakmar, E. et al. Asphyxiated neonates who received active therapeutic hypothermia during transport had higher rates of hypocapnia than controls. Acta Paediatr. 107, 1902–1908 (2018).
Iwata, O. et al. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann. Neurol. 58, 75–87 (2005).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670 (2005).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Takenouchi, T., Iwata, O., Nabetani, M. & Tamura, M. Therapeutic hypothermia for neonatal encephalopathy: JSPNM & MHLW Japan Working Group Practice Guidelines Consensus Statement from the Working Group on Therapeutic Hypothermia for Neonatal Encephalopathy, Ministry of Health, Labor and Welfare (MHLW), Japan, and Japan Society for Perinatal and Neonatal Medicine (JSPNM). Brain Dev. 34, 165–170 (2012).
Thoresen, M. & Whitelaw, A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 106, 92–99 (2000).
Davies, P. & Maconochie, I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg. Med. J. 26, 641–643 (2009).
Kreimeier, U. Pathophysiology of fluid imbalance. Crit. Care 4, S3–S7 (2000).
Nestaas, E. & Walsh, B. H. Hypothermia and cardiovascular instability. Clin. Perinatol. 47, 575–592 (2020).
Sarkar, S. et al. Esophageal and rectal temperatures as estimates of core temperature during therapeutic whole-body hypothermia. J. Pediatr. 162, 208–210 (2013).
Tsuda, K. et al. Therapeutic hypothermia for neonatal encephalopathy: a report from the first 3 years of the Baby Cooling Registry of Japan. Sci. Rep. 7, 39508 (2017).
Shibasaki, J. et al. Outcomes related to 10-min Apgar scores of zero in Japan. Arch. Dis. Child. Fetal Neonatal Ed. 105, 64–68 (2020).
Acknowledgements
The authors are grateful to the staff of participating centres for their contribution to the data collection, the infants and their parents for sharing the clinical information. This work was supported by the Japan Society of Perinatal and Neonatal Medicine, and the Ministry of Health, Labour and Welfare, Japan (H27-001, Special research in perinatal medicine). K.T. is funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research 18K15722). J.S. was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C20K08247). S.I. was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C18K09955). O.I. was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research A20H00102).
Author information
Authors and Affiliations
Consortia
Contributions
K.T., S.I., M.N. and O.I. designed the study and the survey items. All authors participated in the data collection. K.T., T. Isayama, S.I. and O.I. performed the statistical analyses. K.T., J.S., T. Isayama, A.T., T.M. and O.I. contributed to the interpretation of the findings. K.T., J.S. and O.I. drafted the manuscript. All authors critically reviewed and revised the manuscript and gave the final approval of the published version. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The ethics committees approved that parental consent was not needed for the registry, since no patient identifier was collected.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tsuda, K., Shibasaki, J., Isayama, T. et al. Body temperature, heart rate and long-term outcome of cooled infants: an observational study. Pediatr Res 91, 921–928 (2022). https://doi.org/10.1038/s41390-021-01502-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01502-w
This article is cited by
-
Three-year outcome following neonatal encephalopathy in a high-survival cohort
Scientific Reports (2022)
-
Admission temperature of very low birth weight infants and outcomes at three years old
Scientific Reports (2022)